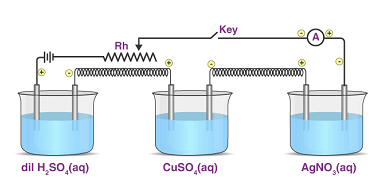
Faraday’s laws of electrolysis
Faraday’s laws of electrolysis are two fundamental laws that describe the quantitative relationship between the amount of electric charge passed through an electrolytic cell and the amount of chemical change that occurs during electrolysis. These laws were developed by the British scientist Michael Faraday in the early 19th century. Faraday’s First Law of Electrolysis states…