Integrated Course AIIMS-SYLLABUS Chemistry syllabus Standard electrode potential
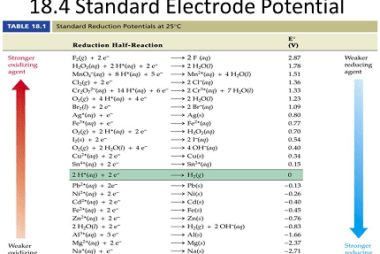
Standard electrode potential Standard electrode potential, often denoted as E°, is a measure of the relative tendency of a half-cell in an electrochemical cell to undergo reduction or oxidation compared to a standard reference electrode. It represents the potential difference between the half-cell and the standard hydrogen electrode (SHE) under standard conditions. The standard electrode…