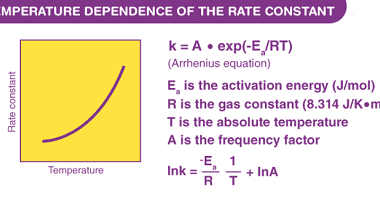
Temperature dependence of rate constant (Arrhenius equation and activation energy)
The temperature dependence of a chemical reaction’s rate constant can be described by the Arrhenius equation, which relates the rate constant to the temperature and the activation energy of the reaction: k = A * exp(-Ea/RT) where k is the rate constant, A is the pre-exponential factor or frequency factor, Ea is the activation energy,…