Elementary ideas of Emulsions
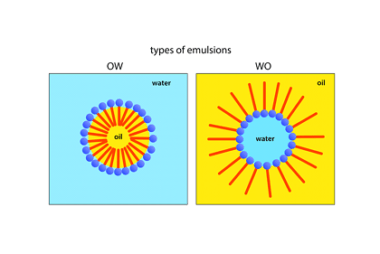
An emulsion is a mixture of two or more immiscible liquids, where one liquid is dispersed throughout the other in small droplets. The dispersed liquid is known as the dispersed phase, while the continuous liquid is known as the continuous phase. The most common example of an emulsion is oil and water, where the oil…