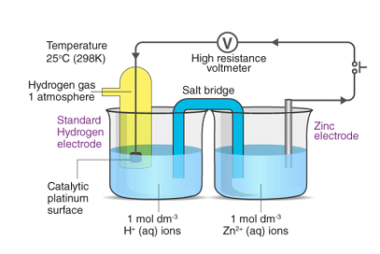
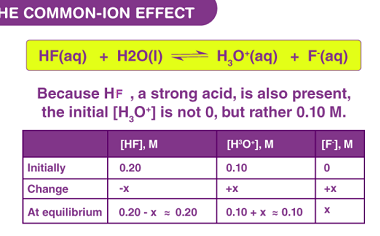
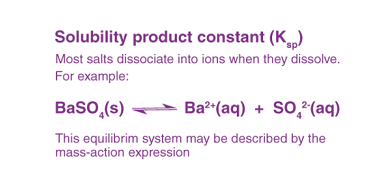
Kohlrausch’s law
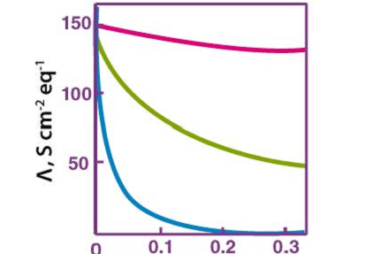
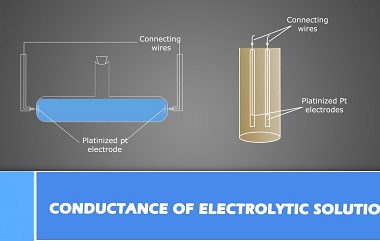
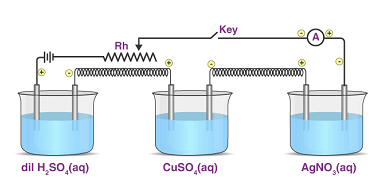
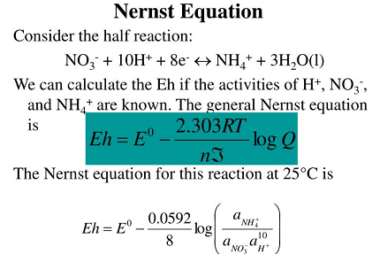
Kohlrausch’s law, also known as Kohlrausch’s displacement law, states that the molar conductivity of an electrolyte can be expressed as the sum of the contributions of its individual ions. In other words, the total conductivity of an electrolyte solution is equal to the sum of the conductivities of the cations and anions present in the…