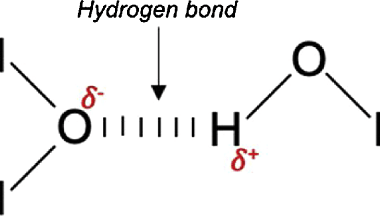
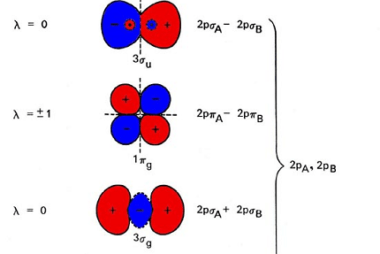
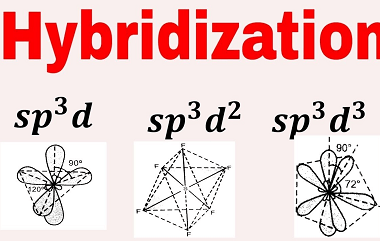
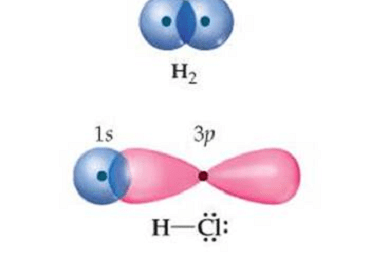
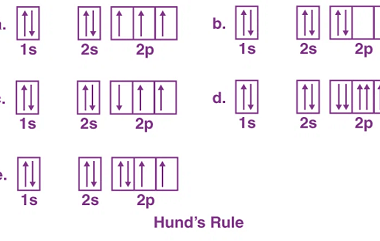
Intensive and Extensive properties
In thermodynamics, properties of matter can be classified as either intensive or extensive. Intensive properties are properties that do not depend on the amount of matter present. Examples of intensive properties include temperature, pressure, density, and specific heat capacity. These properties are useful in describing the state of a system and can be used to…