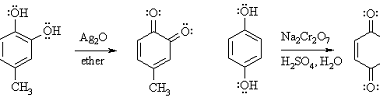
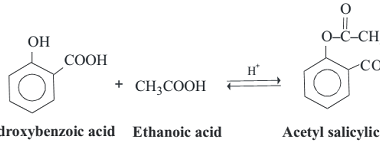
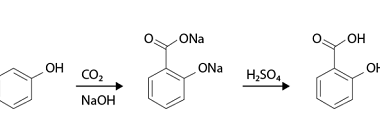
Oxidation
Phenols can undergo different types of oxidation reactions, depending on the conditions and reagents used. Here are a few examples: Overall, the choice of oxidation method depends on the desired products and the specific application. What is Required Phenols Oxidation The requirements for phenols oxidation depend on the specific method used. Here are some general…