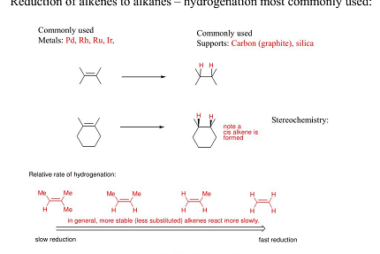
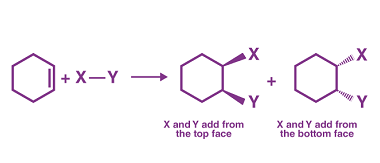
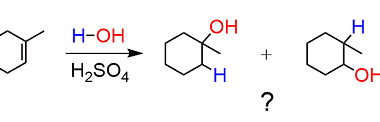
Electrophilic addition reactions of alkenes with X2, HX, HOX, (X=halogen)
Electrophilic addition reactions are the most common reactions of alkenes. When an alkene reacts with an electrophile, the double bond of the alkene is broken and two new sigma bonds are formed. The most common electrophilic addition reactions of alkenes are with halogens, hydrogen halides, and halohydrins. Example reaction: C2H4 + Br2 → C2H4Br2 Example…