Group 16 Halogen
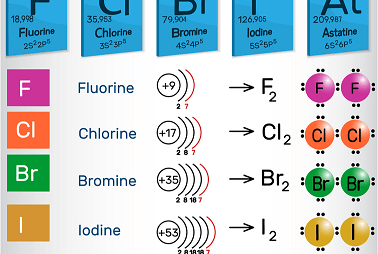
The incandescent lamp (/ˈhælədʒən, ˈheɪ-, – loʊ-, – ˌdʒɛn/) are a gathering in the occasional table comprising of six synthetically related components: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts), however some authors[who?] would reject tennessine as its science is obscure and is hypothetically expected to be more similar to…