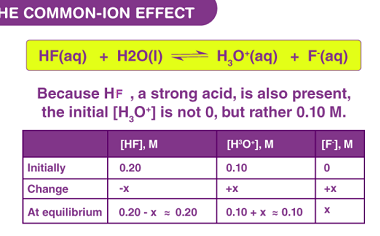
Common ion effect
The common ion effect is a phenomenon in which the solubility of a slightly soluble salt is decreased by the presence of a common ion in the solution. This effect is due to the principle of Le Chatelier’s principle, which states that a system at equilibrium will shift to counteract any stress placed upon it.…