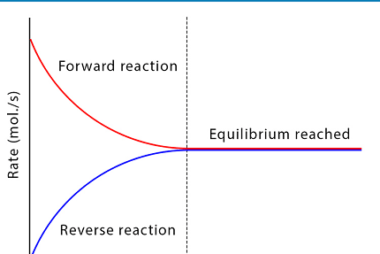
Le Chatelier’s principle (effect of concentration, temperature and pressure)
Le Chatelier’s principle is a fundamental concept in chemistry that describes how a chemical system responds to changes in its environment, such as changes in concentration, temperature, and pressure. The principle states that when a chemical system at equilibrium is subjected to a change in one of these factors, the system will adjust to partially…