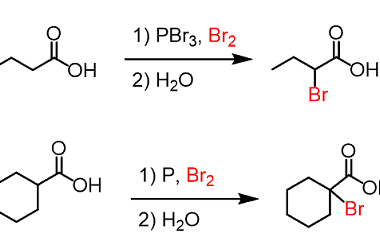
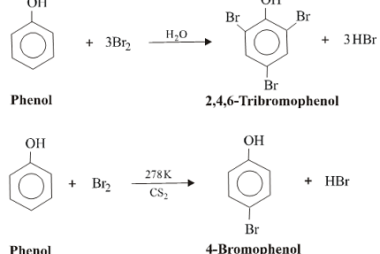
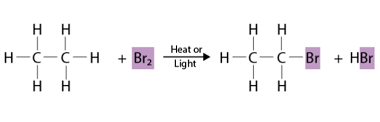
Halogenation
Carboxylic acids are not usually halogenated directly as they are not very reactive towards halogens. However, there are a few methods for the halogenation of carboxylic acids. One method involves the use of phosphorus halides, such as phosphorus tribromide (PBr3) or phosphorus pentachloride (PCl5), to convert the carboxylic acid into an acyl halide. The reaction…