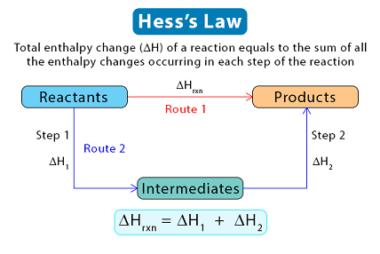
Hess’s law
Hess’s law is a fundamental principle in chemistry that states that the total enthalpy change of a chemical reaction is independent of the pathway between the initial and final states. In other words, if a reaction can occur by multiple paths, the change in enthalpy will be the same regardless of the specific path taken.…