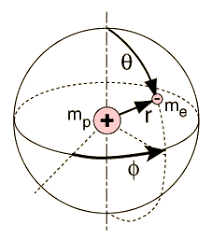
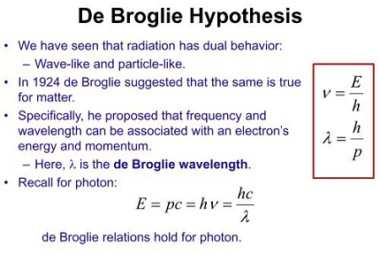
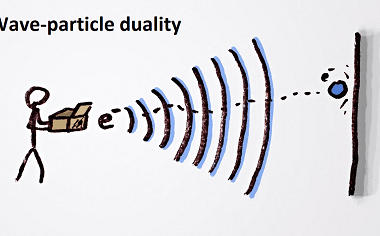
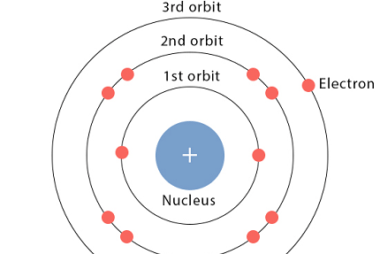
Energies
Energy is a fundamental concept in physics, and it refers to the ability of a system to do work. There are various forms of energy, including: These different forms of energy can be converted from one form to another, and the total amount of energy in a closed system is conserved, meaning it cannot be…