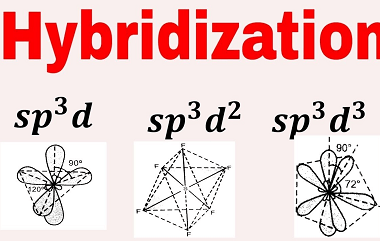
Hybridisation involving s, p and d orbitals only
Hybridization is a concept in chemistry where atomic orbitals combine to form hybrid orbitals that have different shapes and energies from the original atomic orbitals. The most common types of hybridization involve s and p orbitals, but d orbitals can also be involved in certain cases. Hybridization involving only s, p, and d orbitals is…