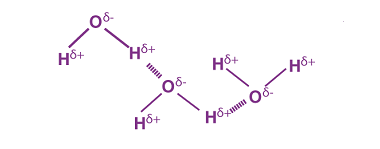
Hydrogen bonding effects
Hydrogen bonding is a type of intermolecular attraction that occurs between a hydrogen atom bonded to a highly electronegative atom (such as nitrogen, oxygen, or fluorine) and a lone pair of electrons on another highly electronegative atom in a nearby molecule. This bonding has several effects: Overall, hydrogen bonding is an important factor that can…