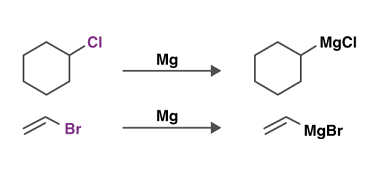
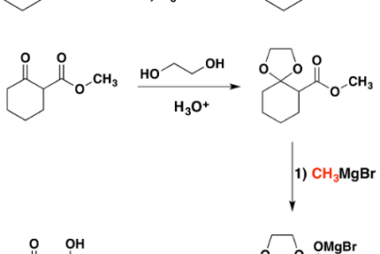
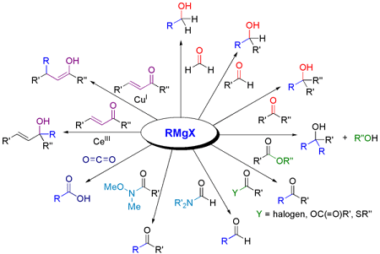
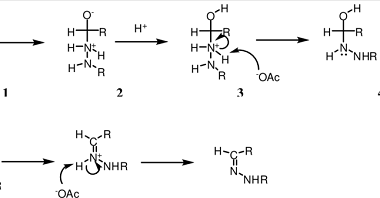
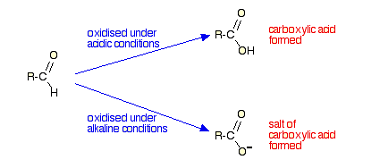
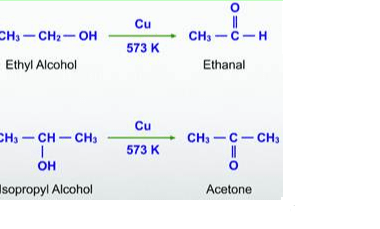
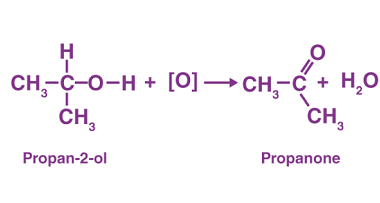
Grignard reagents
Grignard reagents are organometallic compounds that contain a carbon atom bonded to a magnesium atom, often represented as RMgX (where R is an alkyl or aryl group and X is a halogen such as Cl, Br, or I). They are named after their discoverer, French chemist Victor Grignard, who was awarded the Nobel Prize in…