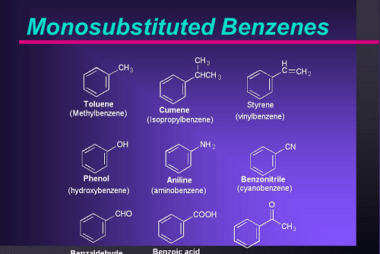
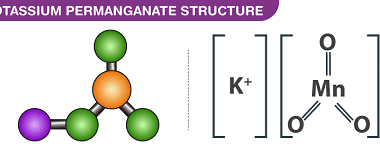
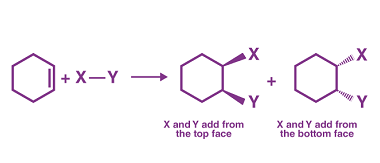
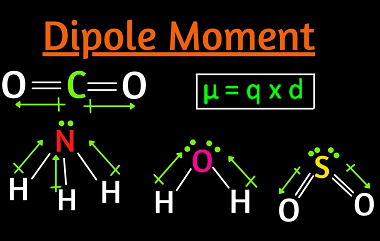
Preparation
Phenols can be prepared through various methods. Here are some common methods: These are just some of the methods that can be used to prepare phenols. The choice of method will depend on the starting material and the desired product. What is Required Phenols Preparation The requirements for preparing phenols can vary depending on the…