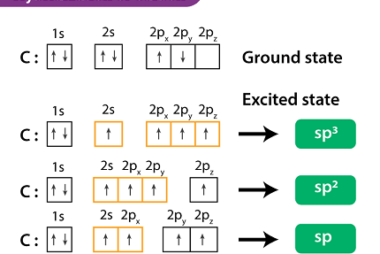
Hybridisation of carbon
Carbon can undergo hybridization to form hybrid orbitals that can participate in chemical bonding. Hybridization of carbon involves the mixing of its valence orbitals, which are the 2s and three 2p orbitals, to form new hybrid orbitals. The most common hybridizations of carbon are sp, sp2, and sp3 hybridizations. The type of hybridization that carbon…