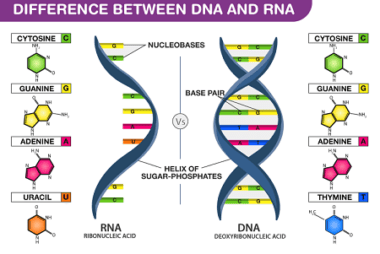
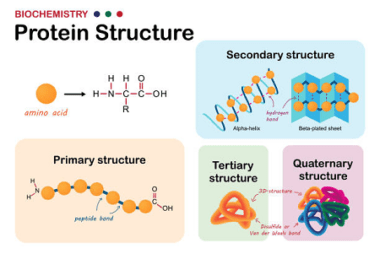
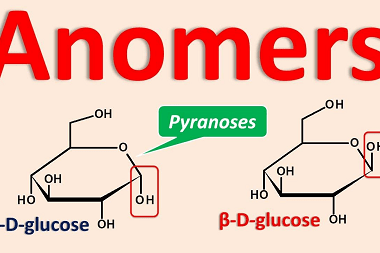
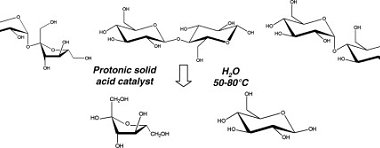
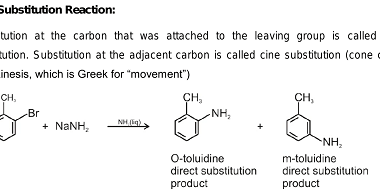
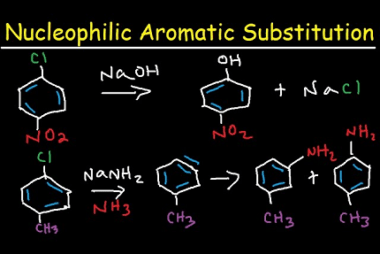
Homo and Copolymers
Homo and copolymers are types of polymers, which are large molecules made up of repeating subunits called monomers. The main difference between homo and copolymers lies in the composition of the monomers. A homopolymer is a polymer made up of only one type of monomer. For example, polyethylene is a homopolymer made up of repeating…