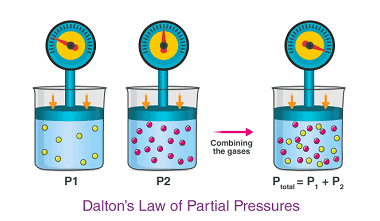
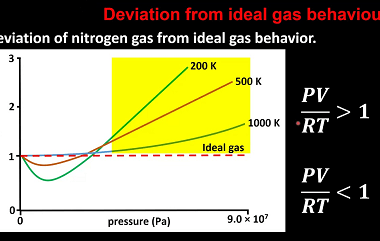
Law of partial pressures
The law of partial pressures, also known as Dalton’s law, states that the total pressure of a mixture of gases is equal to the sum of the partial pressures of the individual gases in the mixture. In other words, if you have a gas mixture made up of two or more gases, the total pressure…