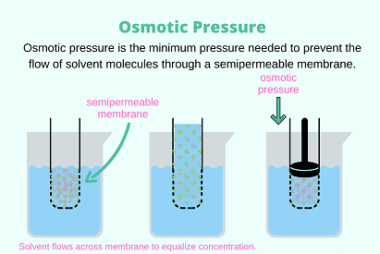
Osmotic pressure
Osmotic pressure is the pressure required to prevent the flow of solvent molecules across a semipermeable membrane, due to the presence of solute molecules. In other words, it is the pressure needed to stop the flow of solvent from a region of low solute concentration to a region of high solute concentration, when the two…