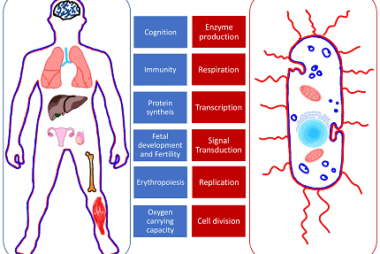
Thermodynamic (iron, copper, zinc)
Thermodynamics is the study of the relationships between energy, work, and heat in a system. Iron, copper, and zinc are all metallic elements with different thermodynamic properties. Iron has a high melting point of 1538°C and a boiling point of 2862°C. It has a specific heat capacity of 0.449 J/g·K and a heat of fusion…