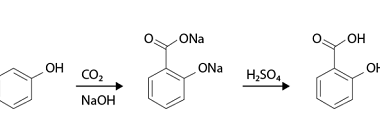
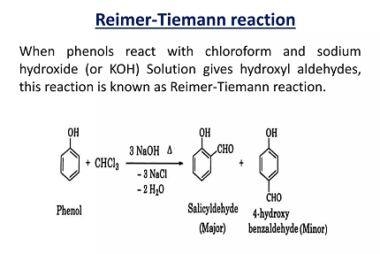
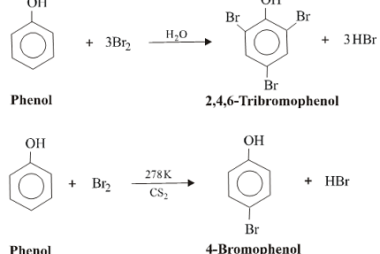
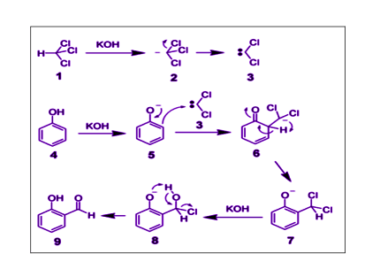
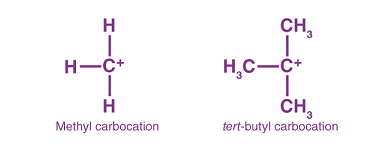Rearrangement reactions of alkyl carbocation
Rearrangement reactions of alkyl carbocations refer to the conversion of one type of carbocation into another via migration of an alkyl group or a hydrogen atom from an adjacent carbon atom. These reactions are common in organic chemistry and are important in the synthesis of various organic compounds. The most common type of rearrangement reaction…