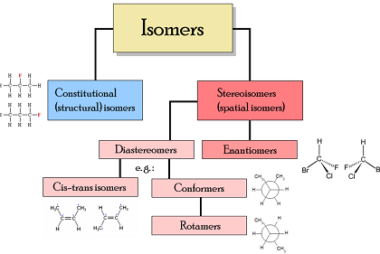
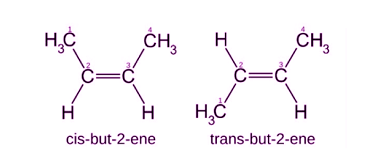
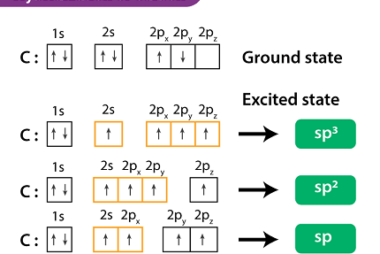
Enantiomers
Enantiomers are a type of stereoisomerism that occurs in organic chemistry when two molecules are non-superimposable mirror images of each other. They have the same chemical and physical properties, except for their effect on plane-polarized light and interactions with other chiral molecules. Enantiomers have a chiral center, which is an atom that is attached to…