Crash Course AIIMS-SYLLABUS Chemistry syllabus Standard electrode potential
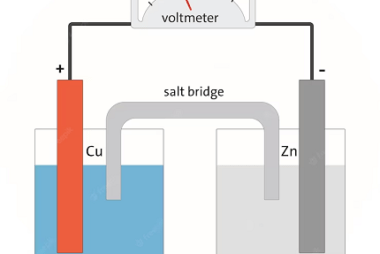
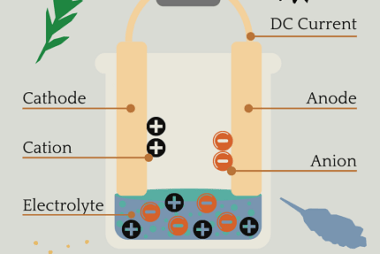
Standard electrode potential The Standard electrode potential is a measure of the potential difference between a half-cell electrode and a standard reference electrode, under standard conditions. It is denoted by E° and is expressed in volts. In the context of the AIIMS (All India Institute of Medical Sciences) syllabus for chemistry, the topic of standard…