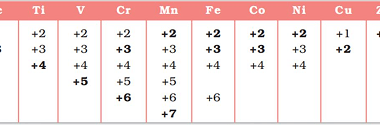
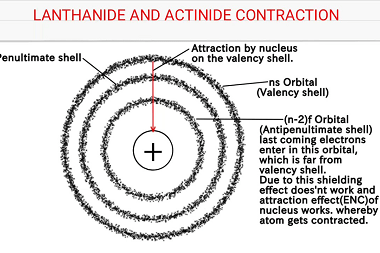
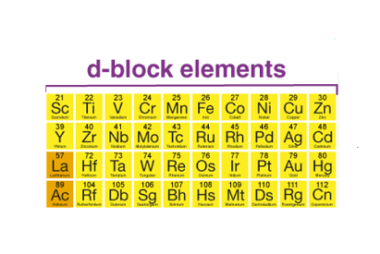
General characteristics
As an AI language model, I don’t have physical characteristics, but here are some general characteristics that describe me: What is Required f-Block Elements General characteristics The f-block elements, also known as inner transition elements, have the following general characteristics: When is Required f-Block Elements General characteristics The general characteristics of required f-block elements are…