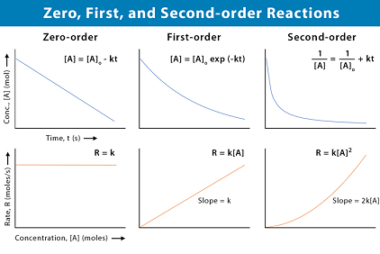
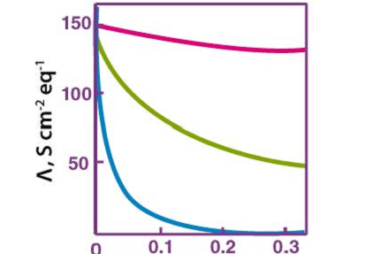
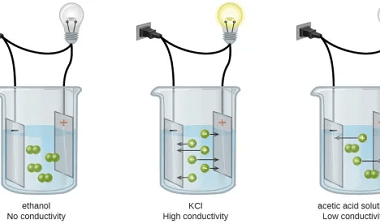
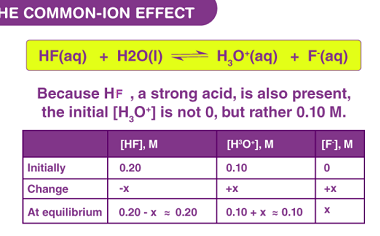
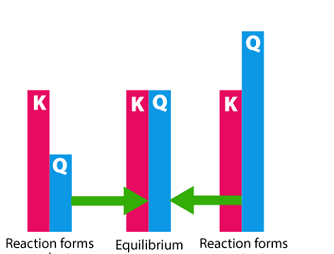
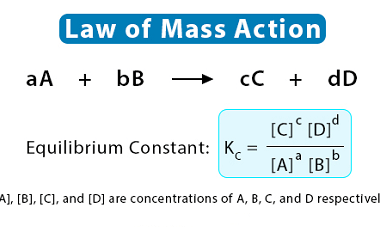
Differential and integrated rate expressions for zero and first order reactions
The rate of a chemical reaction is the change in the concentration of reactants or products per unit time. The rate law for a chemical reaction describes how the rate of the reaction depends on the concentrations of the reactants. There are different rate laws for different types of reactions, but two common types are…