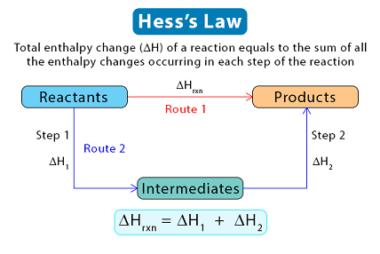
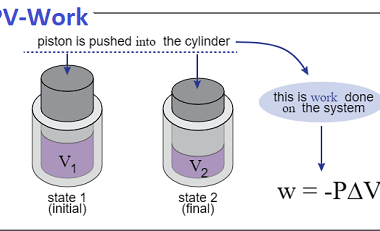
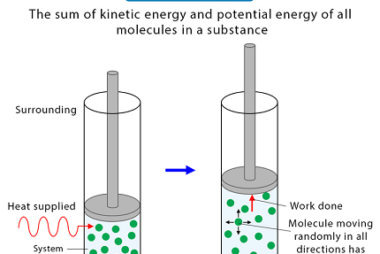
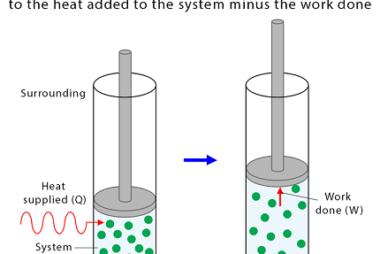
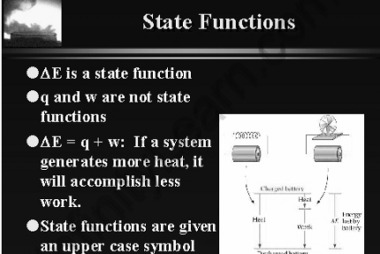
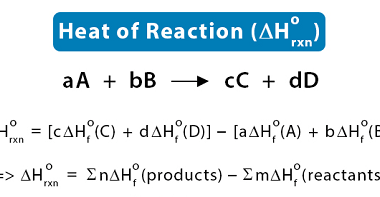Entropy
Entropy is a concept from thermodynamics that refers to the degree of disorder or randomness in a system. In statistical mechanics, it is often defined as the number of microstates (arrangements of particles or energy levels) that correspond to a given macrostate (observable properties like temperature, pressure, or volume). The greater the number of microstates,…