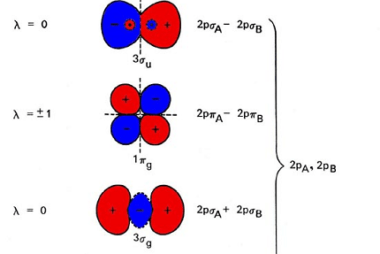
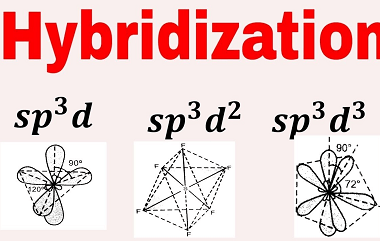
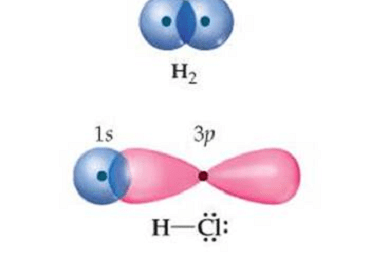
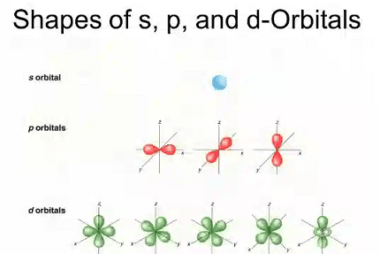
Angular
Angular is a popular open-source web application framework developed and maintained by Google. It is a TypeScript-based framework for building dynamic, single-page web applications (SPAs). Angular follows the Model-View-Controller (MVC) architectural pattern and provides a set of tools and features for building complex and scalable web applications. Some key features of Angular include: Angular is…