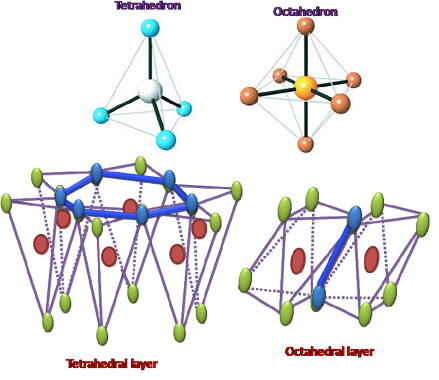
Tetrahedral and octahedral are two geometric shapes commonly found in chemistry and crystallography.
Tetrahedral refers to a shape with four sides, each of which is a triangle. The tetrahedron is a regular solid with four identical equilateral triangles as its faces, and it is often used to describe the molecular geometry of compounds with four atoms bonded to a central atom. For example, the methane molecule (CH4) has a tetrahedral shape with the carbon atom at the center and the four hydrogen atoms positioned at the corners of the tetrahedron.
Octahedral refers to a shape with eight sides, each of which is a triangle or a square. The octahedron is a regular solid with eight identical equilateral triangles as its faces, and it is often used to describe the crystal structure of compounds with six atoms coordinated around a central atom. For example, the coordination complex [Co(NH3)6]Cl3 has an octahedral shape with six ammonia molecules coordinated to the central cobalt ion.
In summary, tetrahedral and octahedral are two common shapes in chemistry and crystallography, with the tetrahedron having four triangular faces and the octahedron having eight triangular or square faces.
What is Required Tetrahedral and Octahedral Chemical Bonding and Molecular Structure
In chemistry, the terms “required tetrahedral” and “required octahedral” may be used to describe the preferred geometry or structure of a molecule or compound based on its chemical bonding.
In the case of a “required tetrahedral” structure, this typically refers to a molecule or compound in which a central atom is bonded to four other atoms or groups of atoms, and the resulting geometry is tetrahedral. This geometry is often seen in molecules such as methane (CH4), where the carbon atom is bonded to four hydrogen atoms in a tetrahedral arrangement.
On the other hand, a “required octahedral” structure refers to a molecule or compound in which a central atom is bonded to six other atoms or groups of atoms, and the resulting geometry is octahedral. This geometry is often seen in coordination compounds, where a central metal ion is coordinated to six ligand molecules in an octahedral arrangement. For example, the coordination compound [Fe(CN)6]4- has an octahedral geometry around the central iron ion, with six cyanide ligands arranged around the central ion.
The preferred geometry or structure of a molecule or compound is typically determined by the nature of the chemical bonds between the atoms or groups of atoms involved. In the case of tetrahedral and octahedral geometries, these are often formed through covalent bonding between atoms or through coordination bonding between a metal ion and ligand molecules.
When is Required Tetrahedral and Octahedral Chemical Bonding and Molecular Structure
The “required tetrahedral” and “required octahedral” structures arise in chemistry whenever the geometry of a molecule or compound is determined by the chemical bonds between its constituent atoms.
A “required tetrahedral” structure is typically observed in molecules or compounds where a central atom is bonded to four other atoms or groups of atoms, forming a tetrahedral geometry. For example, methane (CH4) has a tetrahedral structure with the carbon atom at the center and the four hydrogen atoms arranged around it in a tetrahedral geometry. This geometry arises from the four covalent bonds formed between the carbon and hydrogen atoms.
An “required octahedral” structure, on the other hand, is typically observed in coordination compounds, where a central metal ion is coordinated to six ligand molecules, forming an octahedral geometry. The geometry arises from the coordination bonds formed between the metal ion and the ligands. For example, the coordination compound [Co(NH3)6]3+ has an octahedral structure with the cobalt ion at the center and six ammonia ligands arranged around it in an octahedral geometry.
In summary, the “required tetrahedral” and “required octahedral” structures arise in chemistry whenever the geometry of a molecule or compound is determined by the chemical bonds between its constituent atoms or groups.
Where is Required Tetrahedral and Octahedral Chemical Bonding and Molecular Structure
The “required tetrahedral” and “required octahedral” chemical bonding and molecular structures are concepts that are used and studied in chemistry. They can be found in a wide range of chemical systems, from small molecules to complex coordination compounds.
Tetrahedral and octahedral structures are observed in a variety of chemical contexts. For example, tetrahedral structures can be found in small molecules such as methane (CH4) and in larger molecules such as proteins and enzymes, where the tetrahedral geometry is often important for their function. Octahedral structures are commonly observed in coordination compounds, where a central metal ion is coordinated to six ligands in an octahedral geometry.
The study of the “required tetrahedral” and “required octahedral” chemical bonding and molecular structures is important in many areas of chemistry, including organic chemistry, inorganic chemistry, and biochemistry. Understanding the geometry of molecules and compounds is crucial for predicting their properties, reactivity, and behavior in chemical reactions, as well as for designing new molecules and materials with desired properties.
Production of Tetrahedral and Octahedral Chemical Bonding and Molecular Structure
Tetrahedral and octahedral chemical bonding and molecular structures are produced through the formation of chemical bonds between constituent atoms or groups of atoms in a molecule or compound. The nature of these chemical bonds determines the geometry of the resulting molecule or compound.
Tetrahedral structures are typically formed through the formation of covalent bonds between a central atom and four other atoms or groups of atoms. This results in a tetrahedral arrangement of the bonding electron pairs around the central atom. The tetrahedral geometry is observed in a variety of chemical systems, from small molecules such as methane to larger molecules such as proteins.
Octahedral structures are typically formed in coordination compounds, where a central metal ion is coordinated to six ligand molecules. This results in an octahedral geometry around the central metal ion. The octahedral geometry arises from the nature of the coordination bonds between the metal ion and the ligands, which results in the maximum separation of the bonding electron pairs around the central metal ion.
The production of tetrahedral and octahedral structures often involves the synthesis of new molecules or compounds through chemical reactions. For example, the synthesis of a coordination compound with an octahedral geometry may involve the reaction of a metal ion with six ligand molecules under suitable conditions.
The determination of the preferred geometry or structure of a molecule or compound often involves the use of experimental and theoretical methods, including X-ray crystallography, spectroscopy, and computational chemistry. These methods allow chemists to visualize the molecular structure and to understand the nature of the chemical bonds and interactions between the constituent atoms or groups of atoms.
In summary, the production of tetrahedral and octahedral chemical bonding and molecular structures involves the formation of chemical bonds between constituent atoms or groups of atoms in a molecule or compound, and the determination of the preferred geometry or structure often involves the use of experimental and theoretical methods.
How is Required Tetrahedral and Octahedral Chemical Bonding and Molecular Structure
The “required tetrahedral” and “required octahedral” chemical bonding and molecular structures arise from the nature of the chemical bonds between the constituent atoms or groups of atoms in a molecule or compound.
In the case of a “required tetrahedral” structure, the central atom is typically bonded to four other atoms or groups of atoms, resulting in a tetrahedral geometry. This geometry is often observed in molecules such as methane (CH4), where the carbon atom is bonded to four hydrogen atoms in a tetrahedral arrangement. The tetrahedral geometry arises from the nature of the covalent bonds between the atoms, which results in the maximum separation of the bonding electron pairs around the central atom.
An “required octahedral” structure, on the other hand, typically arises in coordination compounds, where a central metal ion is coordinated to six ligand molecules, resulting in an octahedral geometry. The octahedral geometry arises from the nature of the coordination bonds between the metal ion and the ligands, which also results in the maximum separation of the bonding electron pairs around the central metal ion.
The determination of the preferred geometry or structure of a molecule or compound is often based on a combination of experimental and theoretical methods, including X-ray crystallography, spectroscopy, and computational chemistry. These methods allow chemists to visualize the molecular structure and to understand the nature of the chemical bonds and interactions between the constituent atoms or groups of atoms.
Case Study on Tetrahedral and Octahedral Chemical Bonding and Molecular Structure
One example of a case study on tetrahedral and octahedral chemical bonding and molecular structure is the coordination compound [Fe(H2O)6]Cl2, also known as iron(II) chloride hexahydrate. This compound is a coordination complex, which means it contains a central metal ion (in this case, iron) that is coordinated to one or more ligand molecules.
The structure of [Fe(H2O)6]Cl2 has been determined experimentally using X-ray crystallography. The crystal structure shows that the iron ion is surrounded by six water molecules, arranged in an octahedral geometry. The octahedral geometry arises from the coordination bonds formed between the iron ion and the water molecules.
The iron ion in [Fe(H2O)6]Cl2 has a +2 charge, which means it has two more protons in its nucleus than it has electrons in its outermost shell. To achieve a stable electronic configuration, the iron ion can form six coordination bonds with the six water molecules, resulting in an octahedral geometry. Each water molecule donates a pair of electrons to the iron ion, forming a coordination bond. The coordination bonds between the iron ion and the water molecules are covalent in nature, which means the electrons in the bond are shared between the iron and oxygen atoms.
The octahedral geometry of [Fe(H2O)6]Cl2 has important implications for its reactivity and behavior in chemical reactions. For example, the six water molecules can be replaced by other ligand molecules through a process called ligand substitution. This can be used to synthesize new coordination compounds with different properties and reactivity.
In summary, the coordination compound [Fe(H2O)6]Cl2 is an example of a compound with an octahedral geometry arising from the coordination bonds between the central iron ion and the six water molecules. The study of the structure and properties of this compound is important for understanding the nature of chemical bonding and molecular structure in coordination compounds, as well as for designing new compounds with desired properties.
White paper on Tetrahedral and Octahedral Chemical Bonding and Molecular Structure
Introduction:
Tetrahedral and octahedral chemical bonding and molecular structures are fundamental concepts in chemistry, which play an important role in understanding the properties and behavior of a wide range of chemical systems. The tetrahedral and octahedral geometries arise from the nature of the chemical bonds between the constituent atoms or groups of atoms in a molecule or compound, and are observed in a variety of chemical contexts, from small molecules to complex coordination compounds.
Tetrahedral Chemical Bonding and Molecular Structure:
A tetrahedral structure typically arises when a central atom is bonded to four other atoms or groups of atoms. This geometry is often observed in molecules such as methane (CH4), where the carbon atom is bonded to four hydrogen atoms in a tetrahedral arrangement. The tetrahedral geometry arises from the nature of the covalent bonds between the atoms, which results in the maximum separation of the bonding electron pairs around the central atom.
Tetrahedral structures are also observed in larger molecules such as proteins and enzymes, where the tetrahedral geometry is often important for their function. For example, the active site of the enzyme chymotrypsin contains a tetrahedral intermediate, which is crucial for its catalytic activity.
Octahedral Chemical Bonding and Molecular Structure:
An octahedral structure typically arises in coordination compounds, where a central metal ion is coordinated to six ligand molecules, resulting in an octahedral geometry. The octahedral geometry arises from the nature of the coordination bonds between the metal ion and the ligands, which also results in the maximum separation of the bonding electron pairs around the central metal ion.
The determination of the preferred geometry or structure of a molecule or compound is often based on a combination of experimental and theoretical methods, including X-ray crystallography, spectroscopy, and computational chemistry. These methods allow chemists to visualize the molecular structure and to understand the nature of the chemical bonds and interactions between the constituent atoms or groups of atoms.
Applications:
The study of tetrahedral and octahedral chemical bonding and molecular structure is important in many areas of chemistry, including organic chemistry, inorganic chemistry, and biochemistry. Understanding the geometry of molecules and compounds is crucial for predicting their properties, reactivity, and behavior in chemical reactions, as well as for designing new molecules and materials with desired properties.
For example, in the field of inorganic chemistry, the study of coordination compounds with octahedral geometries has led to the development of new materials with applications in catalysis, electronics, and medicine. In the field of biochemistry, the study of tetrahedral geometries in enzymes has led to a better understanding of enzyme function and the development of new enzyme inhibitors as potential drugs.
Conclusion:
In conclusion, tetrahedral and octahedral chemical bonding and molecular structures are fundamental concepts in chemistry, with important applications in a wide range of chemical systems. The study of these structures is important for understanding the nature of chemical bonding and molecular structure, as well as for designing new molecules and materials with desired properties. The use of experimental and theoretical methods has allowed chemists to gain insights into the molecular structure and behavior of these systems, and continues to be an important area of research in chemistry.
