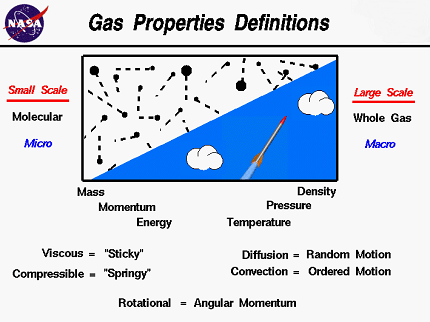
Gases and liquids are two states of matter that exhibit different properties based on their molecular characteristics.
Gases are composed of molecules that are far apart and in constant random motion, and they tend to expand to fill any container they are placed in. The properties of gases are affected by several factors, including temperature, pressure, and volume. Increasing the temperature of a gas tends to increase its average kinetic energy, causing it to expand and decrease in density. Increasing the pressure of a gas tends to decrease its volume and increase its density. As for the effects on specific properties of gases, for instance, increasing the pressure of a gas can increase its solubility in a liquid, while decreasing the temperature of a gas can cause it to condense into a liquid.
On the other hand, liquids are composed of molecules that are closer together than in gases, allowing for more intermolecular forces to exist between them. Liquids are relatively incompressible and tend to take the shape of their container. The properties of liquids are affected by several factors, including temperature, pressure, and intermolecular forces. Increasing the temperature of a liquid tends to increase its average kinetic energy, causing it to expand and decrease in density. Increasing the pressure of a liquid tends to increase its density and decrease its volume. As for the effects on specific properties of liquids, for instance, increasing the temperature of a liquid can decrease its viscosity, while increasing its pressure can increase its boiling point.
What is Required Their effect on properties Gases and Liquids
Understanding the effect of different factors on the properties of gases and liquids requires a basic understanding of the physical and chemical characteristics of these states of matter, as well as an understanding of the factors that affect their behavior. Some of the key factors that can affect the properties of gases and liquids include:
- Temperature: Increasing the temperature of a gas or liquid tends to increase its kinetic energy, which can affect its density, viscosity, and other physical properties.
- Pressure: Changing the pressure on a gas or liquid can affect its volume, density, and other physical properties. Increasing the pressure of a gas can increase its solubility in a liquid, while increasing the pressure of a liquid can increase its boiling point.
- Intermolecular forces: Liquids have stronger intermolecular forces than gases, which can affect their viscosity, surface tension, and other properties.
- Molecular size and shape: The size and shape of molecules in a gas or liquid can affect their behavior, such as how they interact with other molecules and how they flow.
- Chemical composition: The chemical composition of a gas or liquid can affect its properties, such as its reactivity, acidity, or basicity.
Understanding how these factors interact to affect the properties of gases and liquids is essential in fields such as chemistry, physics, and engineering.
Who is Required Their effect on properties Gases and Liquids
The understanding of the effect of different factors on the properties of gases and liquids is required by a variety of professionals across different fields, including:
- Chemists: Chemists study the chemical composition and properties of gases and liquids, including their reactivity, acidity, basicity, and other chemical properties.
- Physicists: Physicists study the physical properties of gases and liquids, including their density, viscosity, surface tension, and other physical properties.
- Engineers: Engineers often work with gases and liquids in designing and optimizing systems for a variety of applications, such as in chemical processing, energy production, and environmental monitoring.
- Environmental scientists: Environmental scientists study the behavior of gases and liquids in natural systems, including their movement through soil, air, and water.
- Medical professionals: Medical professionals often work with gases and liquids in medical applications, such as in the administration of anesthesia and in the development of drug delivery systems.
In short, a wide range of professionals across different fields require an understanding of the effect of different factors on the properties of gases and liquids in order to perform their work effectively.
When is Required Their effect on properties Gases and Liquids
The understanding of the effect of different factors on the properties of gases and liquids is required in many different contexts and situations. Some common examples include:
- In chemistry and physics research: Chemists and physicists need to understand the properties of gases and liquids in order to conduct research into their behavior, interactions, and properties.
- In engineering design: Engineers need to understand the properties of gases and liquids in order to design and optimize systems for a variety of applications, such as in chemical processing, energy production, and environmental monitoring.
- In medical applications: Medical professionals need to understand the properties of gases and liquids in order to develop and administer treatments and medications, such as anesthesia and drug delivery systems.
- In environmental monitoring and analysis: Environmental scientists need to understand the properties of gases and liquids in order to study and monitor their movement through soil, air, and water, and to assess their impact on the environment.
- In everyday life: Understanding the properties of gases and liquids is also important in everyday life, for example in cooking and baking, where the properties of gases are important in understanding the behavior of rising agents like yeast or baking powder, and the properties of liquids are important in understanding the behavior of ingredients like water and oil.
In short, the understanding of the effect of different factors on the properties of gases and liquids is required in a wide range of contexts and situations, from scientific research to everyday life.
Where is Required Their effect on properties Gases and Liquids
The understanding of the effect of different factors on the properties of gases and liquids is required in a variety of settings and environments. Some common examples include:
- Laboratories: In research and development laboratories, scientists and engineers work with gases and liquids to conduct experiments and develop new technologies. Understanding the properties of these substances is essential for success in these environments.
- Manufacturing plants: Many manufacturing processes involve the use of gases and liquids, such as in chemical processing and energy production. Engineers and technicians working in these environments must understand the properties of these substances to optimize production processes and ensure safety.
- Medical facilities: Medical professionals work with gases and liquids in a variety of settings, from hospitals to research laboratories. Understanding the properties of these substances is essential for developing and administering treatments and medications safely and effectively.
- Environmental monitoring sites: Environmental scientists and technicians work in a variety of settings to monitor the behavior and movement of gases and liquids in the environment. Understanding the properties of these substances is essential for assessing their impact on natural systems and developing strategies to mitigate negative effects.
- Kitchens and food production facilities: Understanding the properties of gases and liquids is important in many culinary settings, from baking to cooking. In food production facilities, understanding the properties of gases and liquids is important for quality control and safety.
In short, the understanding of the effect of different factors on the properties of gases and liquids is required in a variety of settings and environments, from scientific research to industrial production, to everyday life.
How is Required Their effect on properties Gases and Liquids
The understanding of the effect of different factors on the properties of gases and liquids is required in a variety of ways, depending on the specific field or application. Some common examples include:
- Conducting experiments: Scientists and engineers often conduct experiments to understand the properties of gases and liquids, such as measuring the density, viscosity, or reactivity of a substance under different conditions. These experiments can be performed in laboratory settings or in the field, depending on the specific application.
- Modeling and simulation: In many cases, it is not practical or possible to conduct experiments to understand the properties of gases and liquids. In these cases, researchers may use modeling and simulation techniques to predict the behavior of these substances under different conditions. This can involve using mathematical equations and computer simulations to model the behavior of gases and liquids.
- Data analysis: Understanding the properties of gases and liquids often involves analyzing data from experiments, simulations, or other sources. This can involve statistical analysis, data visualization, and other techniques to identify patterns and trends in the data.
- Design and optimization: Engineers and designers often use their understanding of the properties of gases and liquids to design and optimize systems for a variety of applications. This can involve selecting materials, designing pipelines and tanks, and optimizing process conditions to achieve desired outcomes.
- Communication and education: The understanding of the properties of gases and liquids is often communicated and taught through scientific papers, textbooks, and other educational materials. Scientists and educators may use visual aids, diagrams, and other tools to help explain complex concepts and make them more accessible to students and other audiences.
In short, the understanding of the effect of different factors on the properties of gases and liquids is required through a variety of methods, including experiments, modeling and simulation, data analysis, design and optimization, and communication and education.
Case Study on Their effect on properties Gases and Liquids
One example of the effect of different factors on the properties of gases and liquids is in the production of natural gas. Natural gas is a fossil fuel that is primarily composed of methane gas. In order to extract natural gas from the ground, a process known as hydraulic fracturing, or “fracking,” is often used.
During the fracking process, a mixture of water, sand, and chemicals is injected into underground rock formations to create fractures. The fractures allow natural gas to escape from the rock and flow to the surface, where it can be collected and processed. Understanding the properties of the gases and liquids involved in this process is essential for optimizing the extraction process and ensuring safety.
One important factor that affects the properties of the gases and liquids involved in fracking is temperature. As the temperature increases, the pressure inside the underground rock formations decreases, which can affect the ability of the natural gas to flow to the surface. Additionally, higher temperatures can increase the volatility of the chemicals used in the fracking process, which can pose a safety risk.
Another important factor that affects the properties of the gases and liquids involved in fracking is pressure. In order for natural gas to flow to the surface, the pressure inside the rock formations must be reduced. However, if the pressure is reduced too much, it can cause the formation of new fractures, which can lead to environmental damage.
The properties of the gases and liquids involved in fracking are also affected by the composition of the fracking fluid. Different chemicals can be used in the fracking fluid to control the viscosity and other properties of the fluid, which can affect the efficiency of the extraction process.
In conclusion, understanding the properties of gases and liquids is essential for optimizing the extraction of natural gas through the fracking process. Factors such as temperature, pressure, and chemical composition can all affect the behavior of these substances, and careful consideration of these factors is essential for ensuring safety and efficiency in the extraction process.
White paper on Their effect on properties Gases and Liquids
Introduction:
Gases and liquids are two distinct phases of matter with different properties that can be affected by various factors. Understanding the effect of different factors on the properties of gases and liquids is important in a variety of industries, including energy, manufacturing, and environmental engineering. This white paper will discuss the properties of gases and liquids and the effect of different factors on these properties.
Properties of Gases:
Gases are characterized by their low density and high compressibility. They also have a relatively low viscosity, which means that they flow easily. The properties of gases are determined by the pressure, temperature, and volume of the gas. The ideal gas law, PV=nRT, describes the relationship between these properties.
One important property of gases is their reactivity. Many gases can react with other substances to form new compounds. For example, oxygen can react with iron to form iron oxide, also known as rust. The reactivity of gases can be affected by temperature, pressure, and the presence of catalysts.
Properties of Liquids:
Liquids are characterized by their high density and low compressibility. They also have a relatively high viscosity, which means that they do not flow as easily as gases. The properties of liquids are determined by their temperature, pressure, and volume. The behavior of liquids can be described using equations such as the Bernoulli equation, which relates the pressure, velocity, and elevation of a liquid in a system.
One important property of liquids is their ability to dissolve other substances. Many liquids can dissolve solids, gases, or other liquids to form solutions. The ability of a liquid to dissolve other substances is affected by temperature, pressure, and the chemical properties of the substances involved.
Effect of Different Factors on Gases and Liquids:
The properties of gases and liquids can be affected by various factors, including temperature, pressure, and the presence of other substances. For example, increasing the temperature of a gas can increase its volume and decrease its density. Similarly, increasing the pressure of a gas can decrease its volume and increase its density.
The presence of other substances can also affect the properties of gases and liquids. For example, the presence of impurities in a liquid can affect its boiling point and freezing point. The presence of catalysts can also affect the reactivity of gases, making them more or less likely to react with other substances.
Conclusion:
Understanding the effect of different factors on the properties of gases and liquids is important in a variety of industries. The properties of gases and liquids can be affected by temperature, pressure, and the presence of other substances, among other factors. By understanding these factors, engineers and scientists can optimize processes and systems to achieve desired outcomes in industries such as energy, manufacturing, and environmental engineering.
