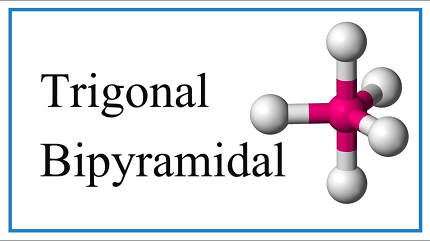
Trigonal bipyramidal is a term used in chemistry to describe the shape of molecules that have five atoms or groups of atoms bonded to a central atom. The shape is named trigonal bipyramidal because it has three equatorial atoms in a plane that form an equilateral triangle, and two axial atoms above and below this plane, forming a vertical axis.
In terms of electron geometry, the central atom has five electron domains, which means that the electron geometry is trigonal bipyramidal. However, the shape of the molecule is determined by the number of atoms or groups of atoms bonded to the central atom, and the number of lone pairs of electrons on the central atom.
If all five positions around the central atom are occupied by bonded atoms, then the molecule has a trigonal bipyramidal shape. Examples of molecules with a trigonal bipyramidal shape include phosphorus pentachloride (PCl5) and sulfur hexafluoride (SF6). If one or more of the positions around the central atom are occupied by lone pairs of electrons, then the shape of the molecule will deviate from ideal trigonal bipyramidal.
What is Required Trigonal bipyramidal
However, if you are referring to a specific compound or situation where a trigonal bipyramidal molecular geometry is required, there are a few examples:
- Phosphorus pentachloride (PCl5) – This compound has a trigonal bipyramidal geometry, with five chlorine atoms bonded to a central phosphorus atom. This geometry is required for the molecule to minimize electron repulsion and achieve the most stable arrangement.
- Sulfur hexafluoride (SF6) – This molecule also has a trigonal bipyramidal geometry, with six fluorine atoms bonded to a central sulfur atom. Like PCl5, this geometry is required to minimize electron repulsion and achieve maximum stability.
- Inorganic chemistry reactions – In some inorganic chemical reactions, a trigonal bipyramidal geometry may be required for the reactants to properly interact with each other and form the desired product.
- Molecular modeling – Trigonal bipyramidal geometry is also a common arrangement used in molecular modeling studies, as it can provide a good representation of how molecules with five bonded atoms can be arranged in space.
Who is Required Trigonal bipyramidal Chemical Bonding and Molecular Structure
For example, some molecules naturally adopt a trigonal bipyramidal geometry in order to minimize repulsion between the electrons in the valence shell. This is the case for molecules such as phosphorus pentachloride (PCl5) and sulfur hexafluoride (SF6), which I mentioned earlier.
In other cases, a trigonal bipyramidal geometry may be required in order to properly interact with other molecules or ions in a chemical reaction. For instance, a molecule with five or six atoms bonded to a central atom may need to adopt this geometry in order to fit properly into a crystal lattice or participate in a specific chemical reaction.
Overall, the use of trigonal bipyramidal geometry in chemical bonding and molecular structure is determined by a combination of factors, including electron repulsion, the properties of the atoms involved, and the specific chemical environment.
When is Required Trigonal bipyramidal
Trigonal bipyramidal geometry is required in molecules or ions when the central atom is surrounded by five bonded atoms or groups of atoms. This arrangement provides the most stable and energetically favorable configuration due to the minimization of repulsion between the valence electrons in the bonds and lone pairs around the central atom.
Some common examples of molecules and ions that adopt trigonal bipyramidal geometry include:
- Phosphorus pentachloride (PCl5) – This molecule has five chlorine atoms bonded to a central phosphorus atom, which adopts a trigonal bipyramidal geometry.
- Sulfur hexafluoride (SF6) – This molecule has six fluorine atoms bonded to a central sulfur atom, which also adopts a trigonal bipyramidal geometry.
- XeF4 (xenon tetrafluoride) – This molecule has four fluorine atoms bonded to a central xenon atom, with two additional lone pairs of electrons on the xenon atom occupying the axial positions, giving it a distorted trigonal bipyramidal shape.
Overall, the trigonal bipyramidal geometry is required in molecules or ions that have five or six atoms bonded to a central atom in order to achieve the most stable and energetically favorable arrangement.
Where is Required Trigonal bipyramidal
Trigonal bipyramidal geometry can be found in various compounds and molecules, both organic and inorganic, in different chemical environments and contexts.
Some examples of where trigonal bipyramidal geometry can be found include:
- Inorganic chemistry – Many inorganic compounds, such as phosphorus pentachloride (PCl5), sulfur hexafluoride (SF6), and xenon tetrafluoride (XeF4), adopt a trigonal bipyramidal geometry due to the nature of their chemical bonding.
- Organic chemistry – Some organic compounds, such as substituted phosphines and certain amino acids, can also adopt a trigonal bipyramidal geometry.
- Crystal structures – Trigonal bipyramidal geometry can also be found in crystal structures, where molecules or ions adopt specific orientations and arrangements based on their bonding interactions.
Overall, trigonal bipyramidal geometry can be found in various chemical and physical environments, and is a common shape for compounds that have five or six atoms bonded to a central atom.
How is Required Trigonal bipyramidal
Trigonal bipyramidal geometry can be achieved in molecules or ions through the arrangement of five atoms or groups of atoms around a central atom. The central atom is typically bonded to three atoms or groups of atoms that lie in a trigonal plane, and two additional atoms or groups of atoms that are positioned along the vertical axis perpendicular to the trigonal plane.
The angle between the three atoms or groups of atoms in the trigonal plane is 120 degrees, while the angle between the two axial atoms or groups of atoms and the three equatorial atoms or groups of atoms is 90 degrees. The bond angles in the trigonal plane and along the vertical axis are therefore different, resulting in a distorted trigonal bipyramidal geometry in some cases.
The specific geometry of a molecule or ion with trigonal bipyramidal geometry is also influenced by the presence of any lone pairs of electrons on the central atom. These lone pairs occupy one of the equatorial positions, which further distorts the geometry of the molecule or ion.
Overall, the trigonal bipyramidal geometry is achieved through the arrangement of atoms or groups of atoms around a central atom, with a specific pattern of bond angles that creates a stable and energetically favorable configuration.
Case Study on Trigonal bipyramidal
One example of a compound with trigonal bipyramidal geometry is phosphorus pentachloride (PCl5). PCl5 is an inorganic compound that is commonly used as a reagent in organic synthesis and as a catalyst in certain reactions.
The central phosphorus atom in PCl5 is surrounded by five chlorine atoms, with three of the chlorine atoms arranged in a trigonal plane and the remaining two chlorine atoms positioned along the vertical axis. The bond angles between the three chlorine atoms in the trigonal plane are 120 degrees, while the bond angles between the axial chlorine atoms and the three equatorial chlorine atoms are 90 degrees.
The geometry of PCl5 is stabilized by the minimization of electron repulsion between the valence electrons in the bonds and lone pairs around the central phosphorus atom. In PCl5, the lone pairs of electrons occupy two of the equatorial positions, which further distorts the geometry of the molecule.
PCl5 is an important reagent in organic synthesis, particularly in the preparation of acid chlorides from carboxylic acids. The reaction between PCl5 and a carboxylic acid results in the formation of an acyl chloride, with the phosphorus atom in PCl5 acting as a Lewis acid and accepting lone pair electrons from the oxygen atom in the carboxylic acid.
Overall, the trigonal bipyramidal geometry in PCl5 is essential for the stability and reactivity of the molecule in chemical reactions, and highlights the importance of molecular geometry in determining the properties and behavior of compounds.
White paper on Trigonal bipyramidal
Introduction:
Trigonal bipyramidal (TBP) geometry is a type of molecular geometry that is commonly observed in chemical compounds containing a central atom surrounded by five other atoms or groups of atoms. The central atom is bonded to three atoms or groups of atoms lying in a trigonal plane and two additional atoms or groups of atoms positioned along the vertical axis perpendicular to the trigonal plane. This type of geometry is observed in a wide variety of compounds, including inorganic and organic molecules, and has important implications for the physical and chemical properties of these compounds.
Bonding in Trigonal Bipyramidal Molecules:
The TBP geometry is a result of the electronic structure of the molecule, which is determined by the bonding between the central atom and the surrounding atoms or groups of atoms. In TBP molecules, the central atom typically has five valence electrons, which can form five covalent bonds with the surrounding atoms. The three atoms in the trigonal plane and the two axial atoms are arranged symmetrically around the central atom, leading to a stable and energetically favorable geometry.
The bond angles in TBP molecules are determined by the repulsion between the electrons in the bonding and non-bonding orbitals of the surrounding atoms. In TBP molecules, the bond angles between the three atoms in the trigonal plane are 120 degrees, while the bond angles between the axial atoms and the three equatorial atoms are 90 degrees. The presence of lone pairs on the central atom can further distort the geometry of the molecule, leading to bond angles that deviate from the ideal values.
Examples of Trigonal Bipyramidal Compounds:
Trigonal bipyramidal geometry is observed in a wide range of compounds, both organic and inorganic. Inorganic compounds such as phosphorus pentachloride (PCl5), sulfur hexafluoride (SF6), and xenon tetrafluoride (XeF4) adopt a TBP geometry due to the nature of their chemical bonding. Organic compounds such as substituted phosphines and certain amino acids can also adopt a TBP geometry.
In PCl5, the central phosphorus atom is surrounded by five chlorine atoms, with three of the chlorine atoms arranged in a trigonal plane and the remaining two chlorine atoms positioned along the vertical axis. The TBP geometry in PCl5 is essential for the stability and reactivity of the molecule in chemical reactions, and highlights the importance of molecular geometry in determining the properties and behavior of compounds.
Applications of Trigonal Bipyramidal Geometry:
The TBP geometry has important applications in various fields of chemistry, including organic synthesis, materials science, and biochemistry. In organic synthesis, TBP molecules are often used as reagents or catalysts due to their stability and reactivity. The TBP geometry is also important in materials science, where it can affect the properties of materials such as polymers and crystals. In biochemistry, TBP molecules are important in the study of proteins and enzymes, which often adopt TBP geometries in their active sites.
Conclusion:
Trigonal bipyramidal geometry is a common molecular geometry observed in a wide range of compounds, both organic and inorganic. The stability and reactivity of compounds with TBP geometry are determined by the electronic structure of the molecule, which is influenced by the bonding between the central atom and the surrounding atoms or groups of atoms. The TBP geometry has important applications in various fields of chemistry, and highlights the importance of molecular geometry in determining the properties and behavior of compounds.