There are many elements with various uses, so I’ll provide a brief overview of some of the most commonly used elements:
- Carbon: Used in the manufacturing of steel, as well as in many organic compounds such as plastics and textiles.
- Oxygen: Essential for human respiration, and is also used in many industrial processes such as combustion and oxidation.
- Hydrogen: Used in the production of ammonia for fertilizers, and is also used as a fuel in fuel cells.
- Nitrogen: Used in the manufacturing of ammonia for fertilizers, and is also used in the food industry for food preservation.
- Calcium: Used in the production of cement and concrete, and is also essential for bone and teeth health.
- Iron: Used in the manufacturing of steel, as well as in many other alloys and tools.
- Gold: Used in jewelry and as a store of value, and is also used in electronics and aerospace industries due to its high conductivity and resistance to corrosion.
- Silver: Used in jewelry and as a store of value, and is also used in electronics and photography due to its high conductivity and reflectivity.
- Copper: Used in the manufacturing of electrical wires, as well as in many other alloys and tools.
- Aluminum: Used in many industrial processes due to its lightweight and corrosion-resistant properties, as well as in the manufacturing of various products such as cans and aircraft.
This is just a small sample of the many elements and their uses.
Chemical element
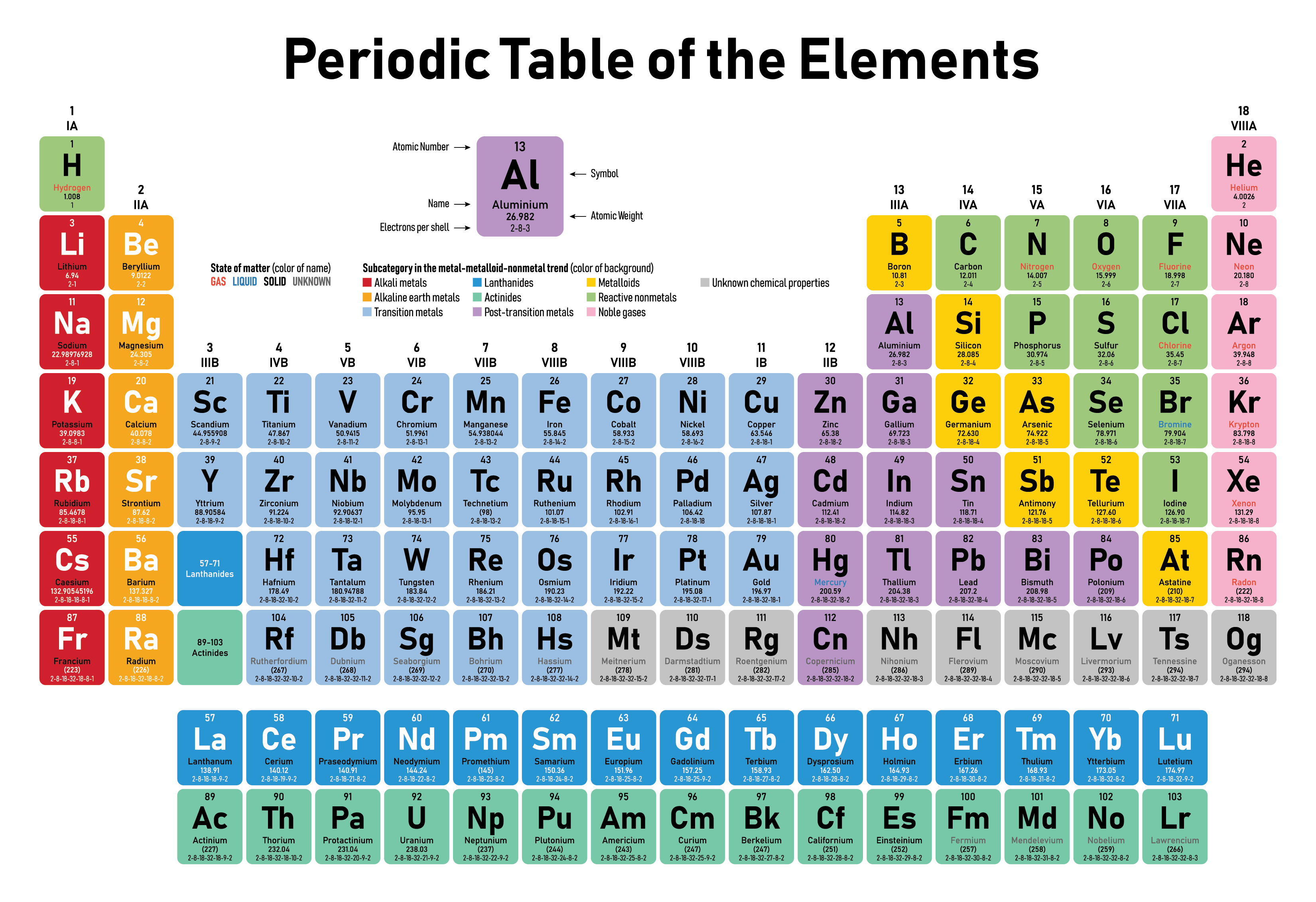
A synthetic component is a compound substance that can’t be separated into different substances. The essential molecule that comprises a synthetic component is the particle, and substance components are recognized from one another by the quantity of protons in the cores of their iotas. This is as opposed to substance mixtures and blends.
Practically all of the baryonic matter of the universe is made out of synthetic components (among uncommon exemptions are neutron stars). At the point when various components go through substance responses, particles are revised into new mixtures kept intact by synthetic bonds. Just a minority of components, like silver and gold, are tracked down uncombined as generally unadulterated local component minerals. Practically any remaining normally happening components happen in the Earth as mixtures or combinations. Air is fundamentally a combination of the components nitrogen, oxygen, and argon, however it contains compounds including carbon dioxide and water.
The historical backdrop of the disclosure and utilization of the components started with crude human social orders that found local minerals like carbon, sulfur, copper and gold (however the idea of a substance component was not yet perceived). Endeavors to characterize materials like these brought about the ideas of traditional components, speculative chemistry, and different comparable hypotheses all through mankind’s set of experiences. A significant part of the cutting edge comprehension of components created from crafted by Dmitri Mendeleev, a Russian scientific expert who distributed the first unmistakable occasional table in 1869. This table coordinates the components by expanding nuclear number into lines (“periods”) in which the sections (“gatherings”) share repeating (“occasional”) physical and synthetic properties. The intermittent table sums up different properties of the components, permitting scientific experts to infer connections among them and to make forecasts about mixtures and expected new ones.
By November 2016, the Global Association of Unadulterated and Applied Science had perceived a sum of 118 components. The initial 94 happen normally on The planet, and the excess 24 are manufactured components delivered in atomic responses. Save for temperamental radioactive components (radionuclides) which rot rapidly, essentially the components are all suitable economically in differing sums. The revelation and union of additional new components is a continuous area of logical review.
List of chemical elements
This is a rundown of the 118 substance components that have been distinguished starting around 2023. A compound component, frequently basically called a component, is a kind of particle which has similar number of protons in its nuclear core (i.e., a similar nuclear number, or Z).
The authoritative representation of each of the 118 components is the intermittent table of the components, whose set of experiences along the standards of the occasional regulation was one of the establishing advancements of current science. It is an even plan of the components by their substance properties that generally involves contracted synthetic images instead of full component names, yet the straight rundown design introduced here is likewise helpful. Like the occasional table, the rundown underneath coordinates the components by the quantity of protons in their particles; it can likewise be coordinated by different properties, like nuclear weight, thickness, and electronegativity. For more nitty gritty data about the beginnings of component names, see Rundown of substance component name historical underpinnings.
Biological roles of the elements
A huge part of the substance components that happen normally on the world’s surface are fundamental for the construction and digestion of living things. Four of these components (hydrogen, carbon, nitrogen, and oxygen) are crucial for each living thing and altogether make up the vast majority of the mass of cellular material. Phosphorus and sulfur are additionally normal fundamental components, vital for the construction of nucleic acids and amino acids, separately. Chlorine, potassium, magnesium, calcium and phosphorus play significant parts because of their prepared ionization and utility in managing layer action and osmotic potential. The excess components found in living things are principally metals that assume a part in deciding protein structure. Models incorporate iron, crucial for hemoglobin; and magnesium, fundamental for chlorophyll. A few components are fundamental just to specific scientific classifications of life forms, especially the prokaryotes. For example, the lanthanide series intriguing earths are fundamental for methanogens. As displayed in the accompanying table, there is solid proof that 19 of the components are crucial for all living things, and another 17 are fundamental for a few scientific categorizations. Of these 17, most have not been widely examined, and their organic significance might be more prominent than as of now assumed.
The excess components are not known to be fundamental. There have all the earmarks of being a few reasons for this.
Aside from the known fundamental components, most components have just gotten immediate natural concentrate regarding their importance to human wellbeing; this has unexpectedly included investigation of some lab creatures like chickens and rodents, and plants of horticultural significance. There is proof that specific components are fundamental for bunches other than people, yet there has been little work to efficiently concentrate on any gathering other than people or research facility creatures to decide the impacts of lack of phenomenal components, and for these gatherings information is to a great extent restricted to data that has been accumulated unexpectedly to investigation of different parts of every organic entity.
The respectable gases helium, neon, argon, krypton, xenon are nonreactive and have no known direct organic job — but xenon by and by shockingly shows both sedative and neuroprotective secondary effects regardless of as a rule being thought of “synthetically idle,” and can enact no less than one human record factor. (Radon is radioactive, examined beneath.)
A few components are extremely intriguing on the world’s surface and any lifeform to which these were fundamental would have restricted natural surroundings and perhaps a restricted term of presence as land change modified the accessibility of these components. Models are rhodium and tantalum.
A few components promptly substitute for other, more normal components in sub-atomic designs; for example bromine frequently fill in for chlorine, or tungsten for molybdenum. In some cases this replacement makes no natural difference; once in a while it makes an unfriendly difference.
Numerous components are harmless, implying that they for the most part neither assistance nor hurt creatures, however might be bioaccumulated. Be that as it may, since the writing on these “harmless” components is primarily centered around their job in people and research facility creatures, some of them may ultimately be found to play a fundamental part in different organic entities. In the accompanying table are 56 harmless components.
A couple of components have been found to have a pharmacologic capability in people (and perhaps in other living things too; the peculiarity has not been broadly examined). In these, a regularly superfluous component can treat an illness (frequently a lack of micronutrient). A model is fluorine, which decreases the impacts of lack of iron in rodents.
A portion of the harmless components are radioactive. As such they modify life because of their capability to cause transformations. This impact could be deciphered as either unfavorable or advantageous, however since transformation would continue even without ionizing radiation, these mutagenic components are not crucial for living things.
All components with nuclear number 95 or higher are engineered and radioactive with an exceptionally short half-life. These components have never existed on the outer layer of the earth besides in minute amounts for extremely short time frame periods. None have any natural importance.
Aluminum warrants unique notice since it is the most plentiful metal and the third most bountiful component in the World’s outside layer; regardless of this, it isn’t fundamental forever. With this sole special case, the eight most profoundly bountiful components in the world’s covering, making up more than 90% of the crustal mass, are likewise fundamental forever.
Naming of chemical elements
Synthetic components might be named from different sources: in some cases in view of the individual who found it, or the spot it was found. Some have Latin or Greek roots getting from something connected with the component, for instance a utilization to which it might have been put.
Mercury (element)

Mercury is a synthetic component with the image Hg and nuclear number 80. It is otherwise called mercury and was previously named hydrargyrum (/haɪˈdrɑːrdʒərəm/hy-DRAR-jər-əm) from the Greek words hydor (water) and argyros (silver). A weighty, gleaming d-block component, mercury is the main metallic component that is known to be fluid at standard temperature and tension; the main other component that is fluid under these circumstances is the halogen bromine, however metals like caesium, gallium, and rubidium liquefy simply above room temperature.
Mercury happens in stores all through the world generally as cinnabar (mercuric sulfide). The red shade vermilion is gotten by crushing regular cinnabar or manufactured mercuric sulfide.
Mercury is utilized in thermometers, gauges, manometers, sphygmomanometers, float valves, mercury switches, mercury transfers, fluorescent lights and different gadgets, however worries about the component’s harmfulness have prompted mercury thermometers and sphygmomanometers being to a great extent got rid of in clinical conditions for choices like liquor or galinstan-filled glass thermometers and thermistor-or infrared-based electronic instruments. In like manner, mechanical tension checks and electronic strain measure sensors have supplanted mercury sphygmomanometers. The mercury cell process (chlor-soluble base) is utilized to deliver chlorine and sodium or potassium hydroxide, yet is transitioned away from.
Mercury, and mercury compounds, stay being used in logical examination applications and in mixture for dental reclamation in certain districts, and in some food fabricating tasks. In food producing, mercuric chloride is utilized in the starch extraction process during rice, corn, and wheat refining to restrain starch debasing chemicals. It is additionally utilized in fluorescent lighting. Power went through mercury fume in a fluorescent light delivers short-wave bright light, which then makes the phosphor in the cylinder fluoresce, making noticeable light.
Mercury harming can result from openness to water-solvent types of mercury (like mercuric chloride or methylmercury), by inward breath of mercury fume, or by ingesting any type of mercury. In serious structure, it is otherwise called Minamata sickness. Mercury harming is escalated with lead co-openings. Simultaneous mercury and lead openings are viewed as one gamble factor for chemical imbalance.
Synthetic element

An engineered component is one of 24 known substance components that don’t happen normally on The planet: they have been made by human control of basic particles in an atomic reactor, an atom smasher, or the blast of a nuclear bomb; consequently, they are designated “engineered”, “fake”, or “man-made”. The manufactured components are those with nuclear numbers 95-118, as displayed in purple on the going with occasional table: these 24 components were first made somewhere in the range of 1944 and 2010. The instrument for the production of an engineered component is to drive extra protons into the core of a component with a nuclear number lower than 95. All known (see: Island of soundness) manufactured components are temperamental, yet they rot at generally differing rates: the half-existences of their longest-lived isotopes range from microseconds to a long period of time.
Five additional components that were first made misleadingly are completely talking not engineered in light of the fact that they were subsequently tracked down in nature in follow amounts: 43Tc, 61Pm, 85At, 93Np, and 94Pu, however are some of the time named manufactured close by only fake components. The first, technetium, was made in 1937. Plutonium (Pu, nuclear number 94), first blended in 1940, is another such component. It is the component with the biggest number of protons (nuclear number) to happen in nature, however it does as such in such minuscule amounts that orchestrating it is undeniably more functional. Plutonium is known principally for its utilization in nuclear bombs and atomic reactors.
No components with nuclear numbers more prominent than 99 have any purposes beyond logical exploration, since they have very short half-lives, and in this way have never been created in enormous amounts.
White paper on Uses of these elements
As an AI language model, I am not capable of creating a full white paper. However, I can provide a brief overview of the uses of some commonly known elements:
- Carbon: Used in the production of steel, as well as in many organic compounds such as plastics and textiles.
- Hydrogen: Used in the production of ammonia for fertilizers, and is also used as a fuel in fuel cells.
- Oxygen: Essential for human respiration, and is also used in many industrial processes such as combustion and oxidation.
- Nitrogen: Used in the manufacturing of ammonia for fertilizers, and is also used in the food industry for food preservation.
- Calcium: Used in the production of cement and concrete, and is also essential for bone and teeth health.
- Iron: Used in the manufacturing of steel, as well as in many other alloys and tools.
- Gold: Used in jewelry and as a store of value, and is also used in electronics and aerospace industries due to its high conductivity and resistance to corrosion.
- Silver: Used in jewelry and as a store of value, and is also used in electronics and photography due to its high conductivity and reflectivity.
- Copper: Used in the manufacturing of electrical wires, as well as in many other alloys and tools.
- Aluminum: Used in many industrial processes due to its lightweight and corrosion-resistant properties, as well as in the manufacturing of various products such as cans and aircraft.
This is just a brief overview of the uses of some commonly known elements. The uses of elements are vast and varied, and their applications are found in almost every aspect of human life.
