Valence is a term used in psychology to describe the emotional content or “positive” or “negative” quality of an individual’s experience, affect or mood. In simpler terms, valence refers to how pleasant or unpleasant an experience or emotion is. Valence is typically measured on a continuum, with positive experiences or emotions (such as joy, happiness, or contentment) at one end and negative experiences or emotions (such as anger, sadness, or fear) at the other end. The concept of valence is often used in research to understand how people respond to different stimuli and how these responses affect their behavior and well-being.
What is Required Valence
“Required valence” is a term that is sometimes used in the field of industrial-organizational psychology to describe the emotional state or “positive” or “negative” quality that is expected or required of individuals in certain job roles or contexts.
For example, a customer service representative may be required to exhibit a positive and cheerful demeanor, even if they are not feeling particularly happy or positive at the moment. This means that the required valence for this job role is positive, and individuals who cannot meet this requirement may not be a good fit for the job.
Similarly, in certain jobs that involve dealing with difficult or stressful situations (such as emergency responders or healthcare professionals), the required valence may be more neutral or focused on maintaining composure and professionalism in the face of challenging circumstances.
Understanding the required valence for a particular job role can be important for organizations when recruiting and selecting employees, as well as in providing training and support to help individuals meet the emotional demands of their work.
When is Required Valence
“Required valence” can be applicable in a variety of situations, particularly in job roles or contexts where emotional expression is a key component of the work.
For example, customer service representatives, salespeople, and other individuals who interact with customers or clients may be required to exhibit a positive and friendly demeanor, even if they are not feeling particularly happy or positive at the moment. Similarly, individuals who work in healthcare, social work, or other service-oriented fields may be required to exhibit empathy and compassion, even when dealing with difficult or emotionally-charged situations.
In addition, certain job roles may require individuals to maintain a more neutral or professional demeanor, particularly in settings where emotional expression may be seen as inappropriate or unprofessional. For example, judges, lawyers, and other legal professionals may be required to maintain a neutral demeanor in the courtroom, regardless of their personal feelings or emotions.
Understanding the required valence for a particular job role can be important for both employers and employees, as it can help ensure that individuals are a good fit for the role and provide guidance for appropriate emotional expression and behavior on the job.
Where is Required Valence
“Required valence” can be found in a variety of settings where emotional expression is an important aspect of the job or role. This can include customer service positions, sales roles, healthcare and social work settings, and other service-oriented fields.
In addition, required valence can also be present in settings where emotional expression may be seen as inappropriate or unprofessional, such as in legal or corporate environments where a neutral or professional demeanor is expected.
Understanding the required valence for a particular job role is important for employers and employees alike, as it can help ensure that individuals are a good fit for the role and provide guidance for appropriate emotional expression and behavior on the job. Employers may provide training and support to help employees meet the emotional demands of their work, while employees may need to develop skills in emotional regulation and expression in order to effectively meet the requirements of their role.
How is Required Valence
“Required valence” refers to the emotional state or “positive” or “negative” quality that is expected or required of individuals in certain job roles or contexts. The required valence can be communicated explicitly through job descriptions, training programs, or organizational policies, or it may be implicit and learned through observation of the behavior of others in the workplace.
There are various ways in which required valence can be established in a job role or context. For example, employers may develop standards or guidelines for emotional expression or behavior on the job, which can help employees understand the expectations for their role. Training programs or workshops may also be provided to help employees develop the necessary emotional skills or to provide guidance on how to express emotions in an appropriate and effective manner.
In addition, the organizational culture and values can also influence the required valence in a workplace. For example, an organization that values positivity and customer service may require a more positive valence from its employees, while an organization that values professionalism and neutrality may require a more neutral valence.
Overall, understanding the required valence in a job role or context is important for both employers and employees, as it can help ensure that individuals are a good fit for the role and provide guidance for appropriate emotional expression and behavior on the job.
Production of Valence
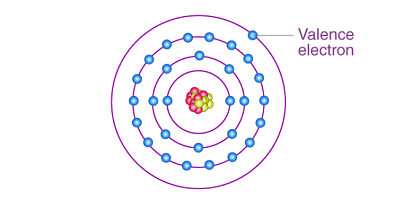
In chemistry, the term “valence” refers to the number of electrons that an atom can give up, accept, or share when it forms a chemical bond with another atom. The production of valence is not a physical process that can be directly manipulated; rather, it is a property of the atoms themselves.
The valence of an atom is determined by its electron configuration, which is the distribution of electrons in the atom’s orbitals. The outermost shell of electrons in an atom, called the valence shell, is the most important in determining its chemical behavior. The number of electrons in the valence shell determines the atom’s valence.
Valence can be thought of as a measure of an atom’s ability to participate in chemical reactions. Atoms with high valence tend to be highly reactive, as they are more likely to either donate or accept electrons in order to achieve a full valence shell. Atoms with low valence are less reactive, as they already have a full valence shell and are less likely to form chemical bonds.
The production of valence is a fundamental aspect of chemistry, as it determines how atoms interact with each other to form molecules and compounds. Understanding the valence of different atoms is essential for predicting chemical reactions and designing new materials.
Case Study on Valence
Let’s consider a case study on the valence of carbon atoms in organic compounds. Carbon is a versatile element that forms the backbone of many organic molecules, such as carbohydrates, lipids, proteins, and nucleic acids. Carbon has four valence electrons, which means it can form up to four covalent bonds with other atoms.
In organic chemistry, the valence of carbon atoms plays a crucial role in determining the structure and function of organic molecules. For example, in alkanes, carbon forms four single bonds with hydrogen or other carbon atoms, resulting in a linear or branched chain structure. In alkenes, carbon forms a double bond with another carbon atom, resulting in a planar structure with restricted rotation.
In addition, the valence of carbon can influence the reactivity and stability of organic compounds. For instance, carbon atoms that are bonded to electronegative atoms, such as oxygen or nitrogen, are more polarized and reactive than those that are bonded to other carbon or hydrogen atoms. This is because the electronegative atoms pull electron density away from the carbon atom, making it more positive and susceptible to nucleophilic attack.
Moreover, the valence of carbon can affect the acidity or basicity of organic compounds. For example, in carboxylic acids, the carbon atom is double-bonded to oxygen and single-bonded to another oxygen, resulting in a carboxyl group (-COOH). The carbon atom in the carboxyl group is electron-deficient and therefore acidic, as it can donate a proton to a base. On the other hand, in amines, the carbon atom is single-bonded to nitrogen and can accept a proton from an acid, making it basic.
In summary, the valence of carbon atoms is a critical aspect of organic chemistry that determines the structure, reactivity, and acidity/basicity of organic compounds. Understanding the valence of carbon and other elements is essential for predicting and designing new organic molecules with desired properties.
White paper on Valence
Introduction:
Valence is a fundamental concept in chemistry that refers to the number of electrons that an atom can share or transfer when it forms a chemical bond with another atom. The valence of an atom is determined by its electron configuration, which is the distribution of electrons in the atom’s orbitals. Valence plays a crucial role in determining the properties and behavior of molecules and compounds, and it is essential for understanding chemical reactions and designing new materials.
Valence in Chemical Bonding:
Valence is closely related to chemical bonding, which is the process by which atoms join together to form molecules and compounds. Chemical bonding occurs when atoms interact with each other through the sharing or transfer of valence electrons. Valence electrons are the outermost electrons in an atom, and they are involved in chemical bonding.
When two atoms come together to form a chemical bond, they can either share valence electrons or transfer them. In covalent bonding, atoms share valence electrons in order to achieve a stable electron configuration. In ionic bonding, atoms transfer valence electrons to form positively and negatively charged ions that are attracted to each other.
Valence in Organic Chemistry:
In organic chemistry, valence plays a crucial role in determining the structure and function of organic molecules. Organic molecules are made up of carbon atoms bonded to other atoms, such as hydrogen, oxygen, nitrogen, and sulfur. Carbon has four valence electrons, which means it can form up to four covalent bonds with other atoms.
The valence of carbon atoms influences the reactivity and stability of organic compounds. For example, carbon atoms that are bonded to electronegative atoms, such as oxygen or nitrogen, are more polarized and reactive than those that are bonded to other carbon or hydrogen atoms. This is because the electronegative atoms pull electron density away from the carbon atom, making it more positive and susceptible to nucleophilic attack.
Moreover, the valence of carbon can affect the acidity or basicity of organic compounds. For example, in carboxylic acids, the carbon atom is double-bonded to oxygen and single-bonded to another oxygen, resulting in a carboxyl group (-COOH). The carbon atom in the carboxyl group is electron-deficient and therefore acidic, as it can donate a proton to a base. On the other hand, in amines, the carbon atom is single-bonded to nitrogen and can accept a proton from an acid, making it basic.
Valence in Inorganic Chemistry:
Valence is also important in inorganic chemistry, where it is used to describe the oxidation state of atoms in compounds. The oxidation state of an atom is a measure of the number of electrons it has gained or lost relative to its neutral state. For example, in the compound FeCl3, the iron atom has a valence of +3, meaning it has lost three electrons and has a positive charge.
The valence of elements in inorganic compounds determines their properties and reactivity. For example, transition metals have variable valence states that enable them to participate in a wide range of chemical reactions, such as oxidation-reduction reactions and coordination chemistry. Valence also plays a crucial role in the formation and stability of ionic compounds, such as salts and minerals.
Conclusion:
In conclusion, valence is a fundamental concept in chemistry that is essential for understanding chemical bonding, organic and inorganic chemistry, and the properties and behavior of molecules and compounds. Valence plays a critical role in determining the structure, reactivity, and stability of chemical compounds, and it is essential for designing new materials and predicting chemical reactions. Understanding the valence of different elements and compounds is crucial for advancing our understanding of the natural world and developing new technologies.
