Wave function
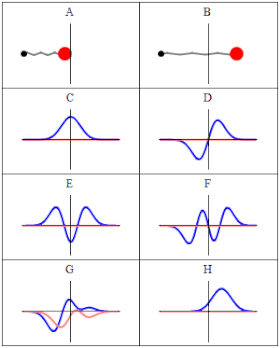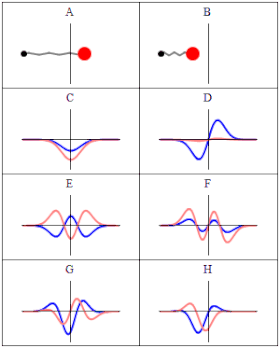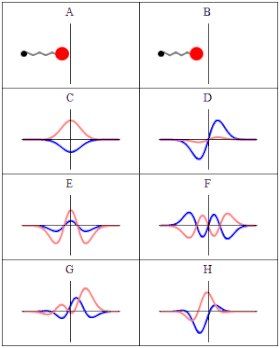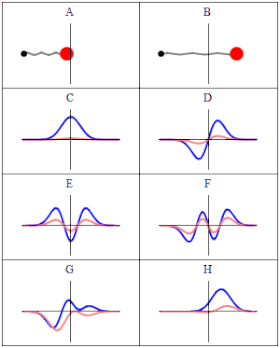
In quantum material science, a wave capability is a numerical depiction of the quantum condition of a secluded quantum framework. The wave capability is a complex-esteemed likelihood plentifulness, and the probabilities for the potential consequences of estimations made on the framework can be gotten from it. The most widely recognized images for a wave capability are the Greek letters ψ and Ψ (lower-case and capital psi, separately).
The wave capability is a component of the levels of opportunity relating to some maximal arrangement of driving observables. When such a portrayal is picked, the wave capability can be gotten from the quantum state.
For a given framework, the decision of which driving levels of opportunity to utilize isn’t remarkable, and correspondingly the space of the wave capability is likewise not exceptional. For example, it could be taken to be a component of all the position directions of the particles over position space, or the momenta of the multitude of particles over energy space; the two are connected by a Fourier change. A few particles, similar to electrons and photons, have nonzero turn, and the wave capability for such particles incorporate twist as an inherent, discrete level of opportunity; other discrete factors can likewise be incorporated, for example, isospin. Whenever a framework has inside levels of opportunity, the wave capability at each point in the constant levels of opportunity (e.g., a point in space) relegates a mind boggling number for every conceivable worth of the discrete levels of opportunity (e.g., z-part of twist) – these qualities are many times shown in a section lattice (e.g., a 2 × 1 segment vector for a non-relativistic electron with turn 1⁄2).
As indicated by the superposition guideline of quantum mechanics, wave capabilities can be added together and increased by complex numbers to frame new wave works and structure a Hilbert space. The inward item between two wave capabilities is a proportion of the cross-over between the comparing actual states and is utilized in the central probabilistic translation of quantum mechanics, the Conceived rule, relating progress probabilities to inward items. The Schrödinger condition decides how wave capabilities develop over the long run, and a wave capability acts subjectively like different waves, for example, water waves or waves on a string, in light of the fact that the Schrödinger condition is numerically a sort of wave condition. This makes sense of the name “wave capability”, and brings about wave-molecule duality. Nonetheless, the wave capability in quantum mechanics portrays a sort of actual peculiarity, actually open to various understandings, which generally contrasts from that of exemplary mechanical waves.
In Conceived’s factual understanding in non-relativistic quantum mechanics, the squared modulus of the wave capability, |ψ|2, is a genuine number deciphered as the likelihood thickness of estimating a molecule as being at a given spot – or having a given energy – at a given time, and potentially having distinct qualities for discrete levels of opportunity. The vital of this amount, over every one of the framework’s levels of opportunity, should be 1 as per the likelihood translation. This overall prerequisite that a wave capability should fulfill is known as the standardization condition. Since the wave capability is perplexing esteemed, just its general stage and relative greatness can be estimated — its worth doesn’t, in confinement, enlighten anything regarding the sizes or headings of quantifiable observables; one needs to apply quantum administrators, whose eigenvalues compare to sets of potential consequences of estimations, to the wave capability ψ and compute the factual disseminations for quantifiable amounts.
Probability density
Probability density is a concept in probability theory that is used to describe the likelihood of a random variable taking on a certain value. It is a function that describes the relative likelihood of different outcomes of a random variable.
The probability density function (PDF) is a non-negative function that integrates to 1 over the entire range of the random variable. The probability density at a particular value of the random variable represents the likelihood of observing that value or any value in a small interval around that value.
The probability density function can be used to calculate the probability of a random variable taking on a value within a certain interval. This is done by integrating the probability density function over that interval.
The probability density function is used to describe the distribution of a random variable. Different probability density functions correspond to different types of distributions, such as the normal distribution, exponential distribution, or Poisson distribution.
Probability density is a fundamental concept in probability theory and has many applications in statistics, physics, engineering, and other fields.
What is Required Atomic Structure Wave function and Probability density
To calculate the wave function and probability density of an atom, we need to solve the Schrödinger equation for the electrons in that atom. This requires knowledge of the electron configuration, which describes how the electrons are distributed among the different energy levels or orbitals of the atom.
Once we know the electron configuration, we can use mathematical models to solve the Schrödinger equation and obtain the wave function and probability density for the atom. The wave function is a complex-valued function that describes the probability amplitude of finding an electron in a particular position, while the probability density is the square of the magnitude of the wave function and represents the probability of finding an electron in a particular position.
The wave function and probability density depend on the energy level and orbital of the electron, and can have different shapes and sizes. For example, the wave function and probability density for the 1s orbital of the hydrogen atom are spherical and have a high probability of finding the electron close to the nucleus, while the wave function and probability density for the 2p orbital of the nitrogen atom are dumbbell-shaped and have two lobes with opposite phases.
In summary, to calculate the wave function and probability density of an atom, we need to know its electron configuration and use mathematical models to solve the Schrödinger equation for the electrons in that atom. The resulting wave function and probability density describe the probability of finding an electron in different positions around the nucleus.
When is Required Atomic Structure Wave function and Probability density
The wave function and probability density of an atom are required whenever we want to study or understand the behavior of the electrons in that atom. This includes a wide range of applications, from understanding the properties of materials and chemical reactions to designing new technologies such as electronic devices.
For example, the wave function and probability density of an atom can be used to determine the energy levels of the electrons and the likelihood of finding an electron in a particular position around the nucleus. This information is important for understanding the electronic structure of atoms and molecules, which in turn determines their chemical properties and reactivity.
In materials science, the wave function and probability density of atoms are used to calculate the electronic and optical properties of materials, such as their conductivity and absorption spectra. In nanotechnology, the wave function and probability density of atoms are used to design and manipulate the properties of nanomaterials and nanodevices, such as quantum dots and nanotubes.
Overall, the wave function and probability density of an atom are essential for understanding and predicting the behavior of electrons in atoms, which has many important applications in various fields of science and technology.
Where is Required Atomic Structure Wave function and Probability density
The wave function and probability density of an atom are mathematical descriptions that exist in the realm of theoretical and computational chemistry and physics. They are not directly observable in the physical world, but rather they are used to make predictions about the behavior of electrons in atoms.
The wave function and probability density are typically calculated using quantum mechanical models, such as the Schrödinger equation or density functional theory (DFT). These models are used to solve for the wave function and probability density of an atom, given its electron configuration and other relevant parameters.
The results of these calculations are typically presented in the form of plots, which show the three-dimensional shape of the probability density around the nucleus of the atom. These plots can help us understand the electronic structure of atoms and molecules, and can be used to make predictions about their chemical properties and reactivity.
Overall, the wave function and probability density of an atom exist in the realm of theoretical and computational chemistry and physics, and are used to make predictions about the behavior of electrons in atoms. They are not directly observable in the physical world, but rather they provide a powerful tool for understanding and predicting the properties and behavior of atoms and molecules.
How is Required Atomic Structure Wave function and Probability density
The wave function and probability density of an atom are determined through the solution of the Schrödinger equation, a partial differential equation that describes the behavior of quantum mechanical systems. The Schrödinger equation contains terms for the energy of the system, the wave function, and the potential energy of the system.
The solution to the Schrödinger equation gives us the wave function, a complex-valued function that describes the probability amplitude of finding an electron in a particular position around the nucleus of the atom. The probability density is then obtained by taking the square of the magnitude of the wave function.
The wave function and probability density depend on the electron configuration of the atom, which describes how the electrons are distributed among the different energy levels or orbitals of the atom. For example, the wave function and probability density for the 1s orbital of the hydrogen atom are spherical and have a high probability of finding the electron close to the nucleus, while the wave function and probability density for the 2p orbital of the nitrogen atom are dumbbell-shaped and have two lobes with opposite phases.
To solve the Schrödinger equation and obtain the wave function and probability density for an atom, we typically use computational methods based on quantum mechanics, such as density functional theory (DFT) or quantum Monte Carlo (QMC) methods. These methods involve approximations and numerical calculations, and can be computationally intensive for larger and more complex systems.
Overall, the wave function and probability density of an atom are determined through the solution of the Schrödinger equation, which depends on the electron configuration of the atom and is typically solved using computational methods based on quantum mechanics.
Case Study on Atomic Structure Wave function and Probability density
One example of a case study involving atomic structure wave functions and probability density is the calculation of the electronic structure of graphene, a two-dimensional carbon material with unique electronic and mechanical properties.
Graphene consists of a single layer of carbon atoms arranged in a hexagonal lattice structure, with each carbon atom bonded to three neighboring atoms. The electronic structure of graphene is determined by the energy levels and wave functions of the carbon atoms, which in turn depend on the geometry and bonding arrangements of the atoms.
To calculate the electronic structure of graphene, researchers typically use computational methods based on density functional theory (DFT), which allows for efficient and accurate calculations of the wave functions and energy levels of large systems. These calculations involve solving the Schrödinger equation for the graphene lattice, which is a complex task due to the large number of atoms and the three-dimensional periodicity of the lattice.
The results of these calculations are typically presented in the form of plots showing the probability density of the electrons in the graphene lattice. These plots reveal the unique electronic properties of graphene, such as its ability to conduct electricity and its unusual electronic band structure.
For example, the probability density plots for the valence band of graphene show that the electrons are localized around the carbon atoms and form a hexagonal pattern, while the probability density plots for the conduction band show that the electrons are delocalized and form a network of interconnected states.
These results provide valuable insights into the electronic properties of graphene and have important implications for its potential applications in electronics, photonics, and other fields. They also demonstrate the power of computational methods based on atomic structure wave functions and probability density for understanding the properties and behavior of complex materials.
White paper on Atomic Structure Wave function and Probability density
Here is a brief white paper on Atomic Structure Wave function and Probability density:
Atomic Structure Wave function and Probability density are essential concepts in the field of quantum mechanics and computational chemistry. The wave function is a complex-valued function that describes the probability amplitude of finding an electron in a particular position around the nucleus of an atom, while the probability density is obtained by taking the square of the magnitude of the wave function.
The wave function and probability density are determined through the solution of the Schrödinger equation, a partial differential equation that describes the behavior of quantum mechanical systems. The solution to the Schrödinger equation gives us the wave function, while the probability density is obtained by taking the square of the magnitude of the wave function.
The wave function and probability density depend on the electron configuration of the atom, which describes how the electrons are distributed among the different energy levels or orbitals of the atom. For example, the wave function and probability density for the 1s orbital of the hydrogen atom are spherical and have a high probability of finding the electron close to the nucleus, while the wave function and probability density for the 2p orbital of the nitrogen atom are dumbbell-shaped and have two lobes with opposite phases.
The wave function and probability density of an atom are typically calculated using quantum mechanical models, such as the Schrödinger equation or density functional theory (DFT). These models are used to solve for the wave function and probability density of an atom, given its electron configuration and other relevant parameters.
The results of these calculations are typically presented in the form of plots, which show the three-dimensional shape of the probability density around the nucleus of the atom. These plots can help us understand the electronic structure of atoms and molecules, and can be used to make predictions about their chemical properties and reactivity.
The wave function and probability density of an atom exist in the realm of theoretical and computational chemistry and physics, and are used to make predictions about the behavior of electrons in atoms. They are not directly observable in the physical world, but rather they provide a powerful tool for understanding and predicting the properties and behavior of atoms and molecules.
In summary, Atomic Structure Wave function and Probability density are essential concepts in the field of quantum mechanics and computational chemistry. They are used to describe the electronic structure of atoms and molecules and provide valuable insights into their properties and behavior.
