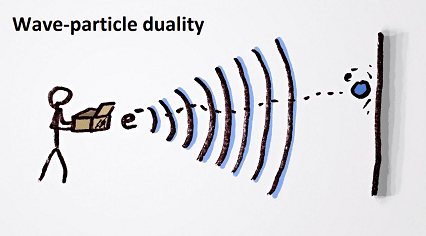
Wave-particle duality is a fundamental concept in physics that describes the nature of matter and light. It refers to the fact that particles, such as electrons and photons, can exhibit both wave-like and particle-like properties, depending on how they are observed and measured.
In some experiments, particles behave as discrete, localized particles with a definite position and momentum, similar to the behavior of billiard balls. However, in other experiments, they behave as waves, with a continuous spread of energy and momentum over space and time, similar to the behavior of waves in water.
This duality is related to the uncertainty principle in quantum mechanics, which states that the more precisely we know a particle’s position, the less precisely we can know its momentum, and vice versa. This means that there is a fundamental limit to the accuracy with which we can simultaneously measure both the position and momentum of a particle.
The wave-particle duality is a central concept in the field of quantum mechanics and has significant implications for the behavior of matter and energy at the atomic and subatomic level. It has also been experimentally demonstrated in numerous experiments, such as the famous double-slit experiment, which shows how particles can interfere with themselves like waves.
What is Required Wave-particle duality
The wave-particle duality of matter and light is required in order to explain certain experimental results that cannot be explained by classical physics. The classical view of nature, which assumes that particles are always particles and waves are always waves, is insufficient to explain the behavior of matter and energy at the atomic and subatomic level.
For example, the photoelectric effect, which is the emission of electrons from a metal surface when it is exposed to light, cannot be explained by classical physics. According to classical physics, the energy of the light should be absorbed by the metal as a continuous wave, and the electrons should be ejected when they have absorbed enough energy. However, the photoelectric effect occurs only when the light is above a certain frequency or energy, indicating that the energy of the light is absorbed by the metal in discrete packets or quanta, as predicted by quantum mechanics.
Similarly, the double-slit experiment, in which electrons or photons are sent through two parallel slits and form an interference pattern on a screen behind the slits, cannot be explained by classical physics. According to classical physics, particles should travel through one of the two slits and form two distinct patterns on the screen, but in reality, they form an interference pattern as if they were waves.
The wave-particle duality is therefore required in order to explain these and other experimental results, and to provide a more complete and accurate understanding of the behavior of matter and energy at the atomic and subatomic level.
Who is Required Wave-particle duality
The concept of wave-particle duality is a fundamental concept in physics that was developed by many scientists over time.
The earliest idea of wave-particle duality can be traced back to the 17th century, when Christiaan Huygens proposed that light consists of waves, and Isaac Newton proposed that light consists of particles. However, it wasn’t until the 20th century that this concept was fully developed and understood with the development of quantum mechanics.
Many famous physicists, such as Max Planck, Albert Einstein, Louis de Broglie, Erwin Schrödinger, and Werner Heisenberg, played important roles in the development of the wave-particle duality concept. Planck introduced the idea of quantization, which suggested that energy could only exist in discrete packets or quanta, while Einstein proposed that light can behave as both a wave and a particle, depending on the experiment. De Broglie introduced the idea of wave-particle duality for matter, suggesting that all particles, including electrons, have wave-like properties.
Schrödinger developed the mathematical framework for wave mechanics, which describes the wave-like behavior of particles, and Heisenberg formulated the uncertainty principle, which describes the limitations on the precision with which we can measure the properties of particles.
Overall, many scientists and physicists have contributed to the development of the concept of wave-particle duality, and it is now a fundamental concept in modern physics.
When is Required Wave-particle duality
The concept of wave-particle duality is required whenever we try to describe the behavior of matter and energy at the atomic and subatomic level. This includes phenomena such as the photoelectric effect, the Compton effect, and the double-slit experiment.
In the photoelectric effect, for example, the wave-particle duality is required to explain why electrons are ejected from a metal surface when it is exposed to light. Classical physics predicts that the energy of the light should be absorbed by the metal as a continuous wave, but in reality, the energy is absorbed by the metal in discrete packets or quanta, as predicted by quantum mechanics.
Similarly, the Compton effect, which is the scattering of X-rays by electrons in a material, can only be explained by wave-particle duality. The scattering of X-rays can be described as both a wave and a particle phenomenon, depending on the experiment, and wave-particle duality is required to explain both aspects of the phenomenon.
The double-slit experiment is another example where wave-particle duality is required. In this experiment, electrons or photons are sent through two parallel slits and form an interference pattern on a screen behind the slits, as if they were waves. The behavior of the particles in this experiment can only be explained by wave-particle duality, where the particles exhibit both wave-like and particle-like properties.
Overall, wave-particle duality is required whenever we try to describe the behavior of matter and energy at the atomic and subatomic level, and it is a fundamental concept in modern physics.
Where is Required Wave-particle duality
The concept of wave-particle duality is required in various fields of physics and technology, including quantum mechanics, particle physics, solid-state physics, and electronics.
In quantum mechanics, wave-particle duality is a fundamental concept that describes the behavior of matter and energy at the atomic and subatomic level. It is essential for understanding the behavior of particles such as electrons, photons, and atoms, and for explaining phenomena such as quantum tunneling and the uncertainty principle.
In particle physics, wave-particle duality is also a fundamental concept that describes the behavior of subatomic particles, including quarks, leptons, and bosons. The wave-particle duality is required to explain the behavior of particles in particle accelerators and in high-energy collisions.
In solid-state physics, wave-particle duality is required to understand the behavior of electrons in materials, including the phenomenon of band structure, which describes the energy levels of electrons in solids.
In electronics, wave-particle duality is required for the development of technologies such as transistors and semiconductors, which rely on the wave-like behavior of electrons in materials.
Overall, the concept of wave-particle duality is required in many areas of physics and technology, and it is a fundamental concept that underpins our understanding of the behavior of matter and energy at the atomic and subatomic level.
How is Required Wave-particle duality
The concept of wave-particle duality is a fundamental concept in quantum mechanics, which describes the behavior of matter and energy at the atomic and subatomic level. It is a way of describing the behavior of particles that exhibit both wave-like and particle-like properties, depending on the experiment.
The wave-like behavior of particles is characterized by their ability to interfere with each other, producing diffraction patterns and interference fringes. The particle-like behavior, on the other hand, is characterized by their ability to be localized in space and time, and to interact with other particles as discrete units.
The wave-particle duality is described mathematically using wave functions, which are mathematical functions that describe the probability of finding a particle at a particular location and time. The wave function describes the wave-like behavior of the particle, while the particle-like behavior is described by the probability distribution of the wave function.
The wave-particle duality is also related to the concept of quantization, which suggests that energy and other physical quantities can only exist in discrete packets or quanta. This concept is essential to understanding the behavior of particles at the atomic and subatomic level and is a key component of quantum mechanics.
Overall, the wave-particle duality is a complex concept that is required to explain the behavior of matter and energy at the atomic and subatomic level. It is a fundamental concept in modern physics, and it is used to describe the behavior of particles in various fields of physics and technology.
Case Study on Wave-particle duality
One of the most famous and significant experiments that illustrate the concept of wave-particle duality is the double-slit experiment.
The double-slit experiment involves shining a beam of electrons or photons through two parallel slits and observing the resulting pattern on a screen behind the slits. The experiment was first performed in the early 19th century by Thomas Young, using light waves, but it has since been performed with electrons and other particles as well.
In the double-slit experiment, when a beam of particles is passed through the slits, they create an interference pattern on the screen behind the slits, as if the particles were waves. This interference pattern consists of a series of bright and dark fringes, indicating areas where the waves are either reinforcing or canceling each other out.
This behavior can only be explained by wave-particle duality, where particles exhibit both wave-like and particle-like properties. The particles behave as waves, creating an interference pattern, but they also behave as particles, being localized and interacting with the detector screen as discrete units.
Moreover, the behavior of the particles in the double-slit experiment is affected by the act of measurement or observation. When the experiment is performed with a detector screen behind each slit to detect which slit the particle passes through, the interference pattern disappears, and the particles behave like classical particles, without exhibiting any wave-like properties.
This phenomenon, known as the observer effect or the measurement problem, is another key concept in quantum mechanics, and it suggests that the act of measurement or observation can affect the behavior of particles.
The double-slit experiment is a significant example of the wave-particle duality, and it has been used to illustrate the concept in various fields of physics and technology. It is an essential experiment for understanding the behavior of matter and energy at the atomic and subatomic level and has contributed to the development of modern physics and technology.
White paper on Wave-particle duality
Introduction:
The wave-particle duality is one of the most fundamental and perplexing concepts in modern physics. It describes the behavior of matter and energy at the atomic and subatomic level and is essential for understanding many phenomena in quantum mechanics, particle physics, solid-state physics, and electronics. This white paper aims to explore the concept of wave-particle duality, its historical background, its mathematical description, and its applications in various fields of physics and technology.
Historical Background:
The concept of wave-particle duality has its roots in the early 19th century, when Thomas Young conducted his famous double-slit experiment, which showed that light could behave as both a wave and a particle. This experiment laid the groundwork for the development of wave optics, which describes the behavior of light waves, and particle optics, which describes the behavior of light as a stream of particles or photons.
In the early 20th century, the wave-particle duality was further developed in the context of quantum mechanics, which describes the behavior of matter and energy at the atomic and subatomic level. Quantum mechanics showed that particles such as electrons, photons, and atoms could exhibit both wave-like and particle-like properties, depending on the experiment. This behavior could not be explained by classical physics, and it led to the development of a new framework for describing the behavior of particles.
Mathematical Description:
The wave-particle duality is described mathematically using wave functions, which are mathematical functions that describe the probability of finding a particle at a particular location and time. The wave function describes the wave-like behavior of the particle, while the particle-like behavior is described by the probability distribution of the wave function.
The wave function is a complex-valued function that satisfies the Schrödinger equation, which is the fundamental equation of quantum mechanics. The Schrödinger equation describes how the wave function evolves over time and how it interacts with other particles and fields.
The wave function can be used to calculate the probability of finding a particle at a particular location and time, as well as the probability of the particle having a particular energy or momentum. This probability distribution can be used to predict the behavior of particles in various experiments and scenarios.
Applications:
The concept of wave-particle duality has numerous applications in various fields of physics and technology. In quantum mechanics, wave-particle duality is essential for understanding the behavior of particles such as electrons, photons, and atoms, and for explaining phenomena such as quantum tunneling and the uncertainty principle.
In particle physics, wave-particle duality is required to explain the behavior of subatomic particles, including quarks, leptons, and bosons. The wave-particle duality is required to explain the behavior of particles in particle accelerators and in high-energy collisions.
In solid-state physics, wave-particle duality is required to understand the behavior of electrons in materials, including the phenomenon of band structure, which describes the energy levels of electrons in solids.
In electronics, wave-particle duality is required for the development of technologies such as transistors and semiconductors, which rely on the wave-like behavior of electrons in materials.
Conclusion:
In conclusion, the wave-particle duality is a fundamental concept in modern physics, which describes the behavior of matter and energy at the atomic and subatomic level. It is a complex concept that is required to explain the behavior of particles in various fields of physics and technology. The concept of wave-particle duality has led to the development of new theories, frameworks, and technologies, and it continues to play a crucial role in advancing our understanding of the universe.
