ZnCl2 is the chemical formula for zinc chloride, and HCl is the chemical formula for hydrochloric acid. When these two substances are combined, they react to form a complex mixture of products.
The reaction between ZnCl2 and HCl is exothermic, meaning that it releases heat as it proceeds. The products of the reaction include hydrogen gas (H2) and zinc chloride dihydrate (ZnCl2·2H2O). The overall reaction can be written as follows:
ZnCl2 + 2HCl → ZnCl2·2H2O + H2
In the presence of excess HCl, the zinc chloride dihydrate can dissolve to form a clear, colorless solution of zinc chloride. This solution can be used in a variety of industrial processes, including as a flux in soldering and welding, as a wood preservative, and as a deodorant.
It is important to note that concentrated HCl is a strong acid and can be corrosive and potentially dangerous if not handled properly. It should be handled with care and in accordance with proper safety protocols.
What is Required Alcohols ZnCl2/Concentrated HCl
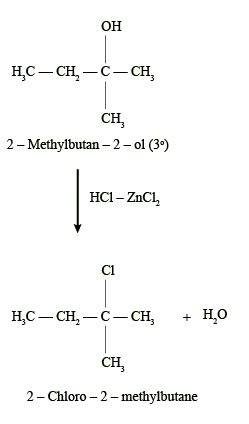
The reaction of alcohols with ZnCl2 and concentrated HCl is a common method for the synthesis of alkyl chlorides. The reaction proceeds through the formation of a carbocation intermediate, which is then attacked by the chloride ion, leading to the substitution of the hydroxyl group with a chloride atom.
The amount of alcohols, ZnCl2, and concentrated HCl required for this reaction would depend on the specific alcohol being used, the desired yield, and the reaction conditions. Generally, the reaction is carried out using equimolar amounts of the alcohol and concentrated HCl, along with a catalytic amount of ZnCl2.
For example, the reaction of ethanol with ZnCl2 and concentrated HCl can be written as follows:
CH3CH2OH + HCl → CH3CH2Cl + H2O
The reaction is typically carried out at room temperature or slightly elevated temperatures and is often conducted in the presence of an inert solvent such as dichloromethane or chloroform. The use of excess HCl or anhydrous HCl gas can also promote the reaction and increase the yield of the desired alkyl chloride.
It is important to note that this reaction can be hazardous and should only be carried out by trained professionals using appropriate safety equipment and procedures.
When is Required Alcohols ZnCl2/Concentrated HCl
The reaction of alcohols with ZnCl2 and concentrated HCl is commonly used in organic chemistry for the synthesis of alkyl chlorides. Alkyl chlorides are versatile organic compounds that are used in a wide range of industrial and pharmaceutical applications, including as solvents, chemical intermediates, and reagents.
The reaction is typically used when there is a need to substitute the hydroxyl group (-OH) in an alcohol molecule with a chloride atom (-Cl). This can be useful in a variety of situations, such as when a particular alkyl chloride is needed for a specific reaction or when the alkyl chloride is a precursor for a more complex organic compound.
In addition to the synthesis of alkyl chlorides, the reaction of alcohols with ZnCl2 and concentrated HCl can also be used in the preparation of alkyl bromides and iodides, by substituting the chloride ion with bromide or iodide ions, respectively.
Overall, the reaction of alcohols with ZnCl2 and concentrated HCl is a useful tool in the toolbox of synthetic organic chemists, allowing them to selectively functionalize alcohols and generate a range of valuable organic compounds.
Where is Required Alcohols ZnCl2/Concentrated HCl
The reaction of alcohols with ZnCl2 and concentrated HCl can be carried out in a laboratory setting, typically in a fume hood due to the potential hazards associated with the use of concentrated HCl. The reaction is typically carried out in a round-bottom flask equipped with a reflux condenser and a stir bar.
The reaction mixture usually consists of the alcohol, ZnCl2, and concentrated HCl in the presence of an inert solvent, such as dichloromethane or chloroform. The mixture is stirred and heated, typically at room temperature or slightly elevated temperatures, for a period of time until the reaction is complete. The progress of the reaction can be monitored using TLC (thin-layer chromatography) or other analytical techniques.
Once the reaction is complete, the organic layer is separated from the aqueous layer, and the desired alkyl chloride is typically purified by distillation or other purification techniques, such as column chromatography or recrystallization.
Overall, the reaction of alcohols with ZnCl2 and concentrated HCl is a standard procedure in organic synthesis, and can be carried out in a laboratory setting by trained professionals using appropriate safety equipment and procedures.
How is Required Alcohols ZnCl2/Concentrated HCl
The reaction of alcohols with ZnCl2 and concentrated HCl involves the substitution of the hydroxyl group (-OH) in the alcohol molecule with a chloride ion (-Cl), resulting in the formation of an alkyl chloride.
The reaction typically requires equimolar amounts of the alcohol and concentrated HCl, along with a catalytic amount of ZnCl2. The reaction is usually carried out in the presence of an inert solvent, such as dichloromethane or chloroform, which helps to facilitate the reaction and minimize side reactions.
The reaction is typically carried out in a round-bottom flask equipped with a reflux condenser and a stir bar. The alcohol and solvent are added to the flask, followed by the addition of the ZnCl2 and concentrated HCl. The mixture is stirred and heated, typically at room temperature or slightly elevated temperatures, until the reaction is complete. The progress of the reaction can be monitored using TLC or other analytical techniques.
Once the reaction is complete, the organic layer is separated from the aqueous layer, and the desired alkyl chloride is typically purified by distillation or other purification techniques, such as column chromatography or recrystallization.
It is important to note that this reaction can be hazardous and should only be carried out by trained professionals using appropriate safety equipment and procedures. The use of concentrated HCl can be particularly dangerous, and precautions should be taken to minimize exposure to the acid and its fumes.
Nomenclature of Alcohols ZnCl2/Concentrated HCl
The reaction of alcohols with ZnCl2 and concentrated HCl results in the formation of alkyl chlorides, which are organic compounds with the general formula R-Cl, where R is an alkyl group.
The nomenclature of alkyl chlorides follows the same rules as for other organic compounds. The parent alkane name is used as the base name, and the suffix “-chloride” is added to indicate the presence of the chloride functional group.
For example, the alkyl chloride formed from the reaction of ethanol with ZnCl2 and concentrated HCl would be called “ethyl chloride” or “chloroethane”.
If the parent alkane has more than one possible position for the functional group, a number is used to indicate the position of the functional group relative to the end of the alkyl chain closest to the functional group. The functional group is then included in the name as a prefix, with the suffix “-yl” indicating the presence of the alkyl chain.
For example, the alkyl chloride formed from the reaction of propanol with ZnCl2 and concentrated HCl, where the chloride is attached to the middle carbon atom of the propane chain, would be called “2-chloropropane” or “sec-propyl chloride”.
It is important to note that the naming conventions for alkyl chlorides and other organic compounds can be complex and depend on a variety of factors, such as the presence of other functional groups or the stereochemistry of the molecule. It is important to consult a reliable reference or textbook for specific naming rules and conventions.
Case Study on Alcohols ZnCl2/Concentrated HCl
One example of the use of alcohols with ZnCl2 and concentrated HCl is in the synthesis of the drug mefloquine, which is used to treat and prevent malaria.
Mefloquine is a 4-quinolinemethanol derivative with the chemical name (RS)-2,8-bis(trifluoromethyl)quinolin-4-yl[(2-piperidyl)methyl]methanol. The synthesis of mefloquine involves several steps, one of which includes the conversion of a hydroxyl group in an intermediate molecule to a chloride using ZnCl2 and concentrated HCl.
In this step, an intermediate molecule with a hydroxyl group is reacted with ZnCl2 and concentrated HCl in dichloromethane to form the corresponding alkyl chloride. The resulting alkyl chloride is then reacted with piperidine to form the desired mefloquine molecule.
The use of ZnCl2 and concentrated HCl in this reaction serves to convert the hydroxyl group to a chloride, which is a necessary step in the synthesis of mefloquine. The reaction must be carefully controlled and monitored to ensure the formation of the desired alkyl chloride, as well as to prevent side reactions or the formation of unwanted byproducts.
Overall, the synthesis of mefloquine is a complex process that involves several steps, including the use of alcohols with ZnCl2 and concentrated HCl to form an alkyl chloride intermediate. This case study highlights the importance of understanding the chemistry and properties of alcohols, as well as the appropriate use of reagents and reaction conditions in organic synthesis.
White paper on Alcohols ZnCl2/Concentrated HCl
Introduction:
Alcohols are a common class of organic compounds that contain a hydroxyl (-OH) functional group. They are used in a variety of industries, including pharmaceuticals, cosmetics, and solvents. The conversion of alcohols to alkyl chlorides is an important reaction in organic chemistry, which can be achieved using ZnCl2 and concentrated HCl. In this white paper, we will discuss the mechanism, applications, and safety considerations of the ZnCl2/Concentrated HCl reaction in the conversion of alcohols to alkyl chlorides.
Mechanism:
The reaction of alcohols with ZnCl2 and concentrated HCl involves the substitution of the hydroxyl group (-OH) in the alcohol molecule with a chloride ion (-Cl), resulting in the formation of an alkyl chloride. The mechanism of the reaction proceeds via an S N 1 pathway, which involves a two-step process.
In the first step, the ZnCl2 catalyzes the protonation of the alcohol, leading to the formation of an oxonium ion. The oxonium ion is an intermediate in the reaction, and it is highly reactive.
In the second step, the chloride ion attacks the carbon atom of the oxonium ion, leading to the formation of an alkyl chloride and a protonated ZnCl2. The protonated ZnCl2 is then deprotonated by the HCl, regenerating the ZnCl2 catalyst and completing the reaction.
Applications:
The ZnCl2/Concentrated HCl reaction is widely used in organic synthesis, particularly in the preparation of alkyl chlorides, which are important intermediates in the synthesis of many organic compounds. Alkyl chlorides can be used in the production of pharmaceuticals, agrochemicals, and surfactants, as well as in the production of polymers and other materials.
For example, the ZnCl2/Concentrated HCl reaction is used in the synthesis of mefloquine, a drug used to treat and prevent malaria. Mefloquine is synthesized by reacting an intermediate molecule with a hydroxyl group with ZnCl2 and concentrated HCl to form the corresponding alkyl chloride. The resulting alkyl chloride is then reacted with piperidine to form the desired mefloquine molecule.
Safety Considerations:
The ZnCl2/Concentrated HCl reaction can be hazardous and should only be carried out by trained professionals using appropriate safety equipment and procedures. The use of concentrated HCl can be particularly dangerous, and precautions should be taken to minimize exposure to the acid and its fumes.
In addition, the reaction can generate heat and gas, which can increase the risk of fire and explosion. It is important to carry out the reaction in a well-ventilated area and to use appropriate protective equipment, such as gloves and eye protection.
Conclusion:
The ZnCl2/Concentrated HCl reaction is an important reaction in organic chemistry, which is widely used in the preparation of alkyl chlorides. The reaction proceeds via an S N 1 pathway, and it is catalyzed by ZnCl2. Alkyl chlorides are important intermediates in the synthesis of many organic compounds, including pharmaceuticals and agrochemicals. However, the reaction can be hazardous and should be carried out by trained professionals using appropriate safety equipment and procedures.