Chemical kinetics is the study of the rates and mechanisms of chemical reactions. In the context of JEE (Main+Advance) integrated course, chemical kinetics is an important topic that is covered in the chemistry syllabus. Here is an overview of the important concepts that you need to know in order to prepare for JEE (Main+Advance) examination:
- Rate of reaction: The rate of a chemical reaction is the change in concentration of reactants or products per unit time. The rate can be expressed as the rate of disappearance of reactants or the rate of appearance of products.
- Factors affecting rate of reaction: There are several factors that can affect the rate of a chemical reaction, including temperature, concentration, surface area, catalysts, and the nature of reactants.
- Rate law: The rate law is an equation that relates the rate of a reaction to the concentrations of the reactants. The rate law is determined experimentally and can be used to predict the rate of the reaction under different conditions.
- Order of reaction: The order of a reaction is the sum of the exponents in the rate law equation with respect to each reactant. The order can be determined experimentally and can be used to predict the effect of changing the concentration of one or more reactants.
- Rate constant: The rate constant is a proportionality constant that relates the rate of a reaction to the concentrations of the reactants. The rate constant is specific to each reaction and can be used to calculate the rate of the reaction at any given concentration.
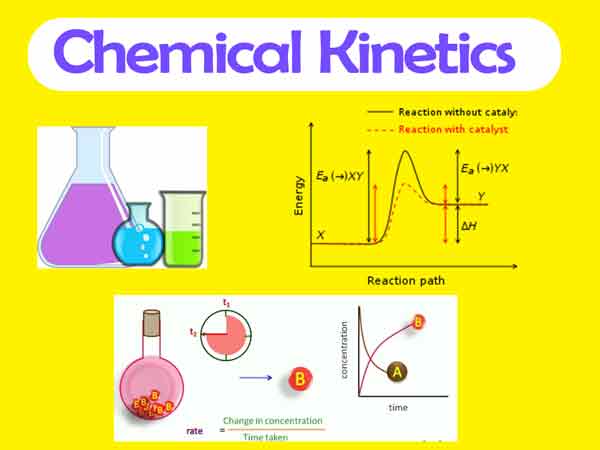
- Activation energy: The activation energy is the minimum energy required for a reaction to occur. The activation energy can be calculated from the rate constant and the temperature of the reaction.
- Reaction mechanisms: The reaction mechanism is the sequence of elementary steps that leads to the overall reaction. The reaction mechanism can be determined experimentally and can provide insight into the reaction pathway and the rate-determining step.
- Arrhenius equation: The Arrhenius equation relates the rate constant to the activation energy and the temperature of the reaction. The Arrhenius equation can be used to calculate the rate constant at any given temperature.
- Catalysis: Catalysis is the process by which a substance called a catalyst increases the rate of a chemical reaction without being consumed in the reaction. Catalysts can work by lowering the activation energy or by providing an alternative reaction pathway.
In summary, chemical kinetics is an important topic that is covered in the JEE (Main+Advance) integrated course. It is essential to understand the key concepts and equations in order to solve problems and predict the behavior of chemical reactions.
History of JEE (Main+Advance) Integrated Course Chemical Kinetics
The Joint Entrance Examination (JEE) is an engineering entrance examination in India that is used for admission to various undergraduate engineering programs across the country. The JEE (Main+Advance) integrated course was introduced in 2015 to streamline the admission process for students who wanted to pursue engineering.
Chemical kinetics has been a part of the JEE (Main+Advance) integrated course syllabus since its inception. The topic has always been an important part of the chemistry section of the examination, as it plays a crucial role in understanding the behavior of chemical reactions and their applications in various fields of engineering.
Over the years, the JEE (Main+Advance) integrated course syllabus has been revised and updated to keep up with the changing trends in engineering education. The chemical kinetics section of the syllabus has also undergone several changes to reflect the latest developments in the field.
Today, the chemical kinetics section of the JEE (Main+Advance) integrated course syllabus covers a wide range of topics, including rate of reaction, order of reaction, rate law, activation energy, reaction mechanisms, and catalysis. The examination typically includes both multiple-choice and numerical-answer questions to test the students’ understanding of these concepts and their ability to apply them to solve problems.
Overall, the inclusion of chemical kinetics in the JEE (Main+Advance) integrated course syllabus highlights the importance of this topic in engineering education and its relevance to real-world applications.
Nature of JEE (Main+Advance) Integrated Course Chemical Kinetics
The JEE (Main+Advance) integrated course syllabus for chemical kinetics covers both the theoretical and practical aspects of the subject. The nature of the course is designed to develop a strong understanding of the fundamental concepts of chemical kinetics and their applications in various fields of engineering.
The course begins with an introduction to the basic concepts of chemical kinetics, including the rate of reaction, order of reaction, rate law, and the factors that affect the rate of reaction. This is followed by a more in-depth study of the reaction mechanisms, activation energy, and catalysis.
The theoretical aspect of the course is supported by practical laboratory experiments, where students learn to measure the rate of chemical reactions under different conditions, determine the order of reactions, and calculate the rate constant. These experiments are designed to develop the students’ practical skills, enhance their understanding of the concepts, and reinforce the theory learned in class.
The JEE (Main+Advance) integrated course syllabus for chemical kinetics also includes a strong emphasis on problem-solving skills. Students are required to solve numerical problems related to chemical kinetics and apply their knowledge to real-world scenarios. This helps them develop their analytical and critical thinking skills and prepares them for the competitive nature of the JEE (Main+Advance) examination.
Overall, the JEE (Main+Advance) integrated course in chemical kinetics is designed to provide students with a solid foundation in the subject and prepare them for a career in engineering or related fields. It emphasizes the importance of understanding the fundamental concepts of chemical kinetics and their practical applications, as well as the development of problem-solving and analytical skills.
Importance of JEE (Main+Advance) Integrated Course Chemical Kinetics
The JEE (Main+Advance) integrated course in chemical kinetics is an important part of the engineering education curriculum for several reasons:
- Understanding of chemical reactions: Chemical kinetics provides a fundamental understanding of how chemical reactions occur, and the factors that influence their rates. This knowledge is essential for engineers in a wide range of fields, including materials science, energy, and pharmaceuticals.
- Optimization of chemical processes: Chemical kinetics helps engineers to optimize chemical processes by controlling the rate and efficiency of chemical reactions. This is critical for industrial processes that rely on chemical reactions, such as the production of chemicals, fuels, and pharmaceuticals.
- Development of new materials: Chemical kinetics plays a key role in the development of new materials, such as advanced polymers, composites, and ceramics. Understanding the kinetics of chemical reactions is essential for optimizing the synthesis and properties of these materials.
- Design of catalysts: Catalysts are critical for enhancing the rate and selectivity of chemical reactions, and chemical kinetics plays a key role in the design and optimization of catalysts. This is essential for industrial processes that rely on catalysis, such as the production of fertilizers and petrochemicals.
- Environmental applications: Chemical kinetics is also important for environmental applications, such as the study of atmospheric chemistry and the design of pollution control systems. Understanding the kinetics of chemical reactions is essential for predicting and controlling the impact of chemical processes on the environment.
Overall, the JEE (Main+Advance) integrated course in chemical kinetics is important for developing the fundamental knowledge and skills that engineers need to solve real-world problems in a wide range of fields. It provides a strong foundation for further study in areas such as materials science, energy, and environmental engineering, and prepares students for a successful career in engineering.
System of JEE (Main+Advance) Integrated Course Chemical Kinetics
The JEE (Main+Advance) integrated course in chemical kinetics is typically structured as follows:
- Introduction to chemical kinetics: The course typically begins with an introduction to the fundamental concepts of chemical kinetics, including reaction rates, rate laws, and reaction order. This section also covers the factors that influence reaction rates, such as temperature, concentration, and catalysts.
- Reaction mechanisms: The course then moves on to a more in-depth study of reaction mechanisms, including elementary steps, intermediates, and transition states. This section also covers topics such as reaction intermediates, activation energy, and the Arrhenius equation.
- Catalysis: Catalysis is a critical topic in chemical kinetics, and the course typically includes a dedicated section on this topic. This section covers topics such as types of catalysts, catalytic mechanisms, and the kinetics of catalyzed reactions.
- Laboratory experiments: The JEE (Main+Advance) integrated course in chemical kinetics typically includes laboratory experiments that allow students to measure the rates of chemical reactions and determine reaction orders and rate constants. These experiments are designed to reinforce the concepts learned in class and develop practical skills.
- Problem-solving: Problem-solving is an important part of the JEE (Main+Advance) integrated course in chemical kinetics. Students are required to solve numerical problems related to chemical kinetics, and apply their knowledge to real-world scenarios.
The course typically includes both theoretical and practical components, and is designed to develop the students’ understanding of the fundamental concepts of chemical kinetics, their practical applications, and their problem-solving skills. By the end of the course, students are expected to have a strong foundation in the subject, and be able to apply their knowledge to real-world problems in engineering and related fields.
Conclusion of JEE (Main+Advance) Integrated Course Chemical Kinetics
In conclusion, the JEE (Main+Advance) integrated course in chemical kinetics is an important part of the engineering education curriculum. It provides students with a fundamental understanding of how chemical reactions occur, and the factors that influence their rates. The course covers topics such as reaction rates, rate laws, reaction mechanisms, and catalysis. Laboratory experiments and problem-solving are also important components of the course, helping students to develop practical skills and apply their knowledge to real-world scenarios.
The importance of chemical kinetics in engineering cannot be overstated. It plays a critical role in the optimization of chemical processes, the development of new materials, the design of catalysts, and environmental applications. Chemical kinetics is also important for the study of atmospheric chemistry and the design of pollution control systems.
The JEE (Main+Advance) integrated course in chemical kinetics provides students with a strong foundation in the subject, preparing them for further study in areas such as materials science, energy, and environmental engineering. It also prepares them for a successful career in engineering or related fields. Overall, the course emphasizes the importance of understanding the fundamental concepts of chemical kinetics, their practical applications, and the development of problem-solving and analytical skills.
Overview of JEE (Main+Advance) Integrated Course Chemical Kinetics
The JEE (Main+Advance) Integrated Course in Chemical Kinetics is a comprehensive course designed to provide students with a thorough understanding of the principles of chemical kinetics, and their practical applications in engineering and related fields. The course covers a wide range of topics, including reaction rates, rate laws, reaction mechanisms, and catalysis. It also includes laboratory experiments and problem-solving exercises, which allow students to apply their knowledge to real-world scenarios.
The course is typically structured to provide students with a strong foundation in the subject. It begins with an introduction to the fundamental concepts of chemical kinetics, including reaction rates and factors that influence them. The course then moves on to more advanced topics, such as reaction mechanisms, catalysis, and laboratory experiments.
Throughout the course, students are encouraged to develop their problem-solving and analytical skills. They are required to solve numerical problems related to chemical kinetics and apply their knowledge to real-world scenarios.
The importance of chemical kinetics in engineering cannot be overstated. It is critical for the optimization of chemical processes, the development of new materials, the design of catalysts, and environmental applications. The JEE (Main+Advance) Integrated Course in Chemical Kinetics prepares students for further study in areas such as materials science, energy, and environmental engineering, and prepares them for a successful career in engineering or related fields.
Overall, the JEE (Main+Advance) Integrated Course in Chemical Kinetics provides students with a strong foundation in the subject, emphasizing the importance of understanding the fundamental concepts of chemical kinetics, their practical applications, and the development of problem-solving and analytical skills.
Types of JEE (Main+Advance) Integrated Course Chemical Kinetics
There are several types of JEE (Main+Advance) integrated courses in chemical kinetics, each designed to meet the specific needs and interests of students. Some common types of courses are:
- Basic Chemical Kinetics: This course covers the fundamental concepts of chemical kinetics, including reaction rates, rate laws, and reaction order. It also covers the factors that influence reaction rates, such as temperature, concentration, and catalysts. The course typically includes laboratory experiments and problem-solving exercises.
- Advanced Chemical Kinetics: This course builds on the fundamental concepts covered in basic chemical kinetics and delves deeper into the topic. It covers topics such as reaction mechanisms, catalysis, and the kinetics of complex reactions. The course also includes advanced laboratory experiments and problem-solving exercises.
- Environmental Chemical Kinetics: This course focuses on the application of chemical kinetics to environmental problems, such as air and water pollution. It covers topics such as atmospheric chemistry, the kinetics of pollutant degradation, and the design of pollution control systems. The course also includes laboratory experiments and problem-solving exercises related to environmental applications.
- Industrial Chemical Kinetics: This course focuses on the application of chemical kinetics to industrial processes. It covers topics such as reaction engineering, reactor design, and the optimization of chemical processes. The course also includes laboratory experiments and problem-solving exercises related to industrial applications.
- Computational Chemical Kinetics: This course focuses on the use of computational methods to study chemical kinetics. It covers topics such as quantum mechanics, molecular dynamics, and kinetic simulations. The course also includes laboratory exercises in computational chemistry.
These are just a few examples of the types of JEE (Main+Advance) integrated courses in chemical kinetics that are available. The specific course offerings may vary by institution, and students are encouraged to explore their options to find the course that best meets their needs and interests.
Structures of JEE (Main+Advance) Integrated Course Chemical Kinetics
The structure of JEE (Main+Advance) integrated course in chemical kinetics can vary depending on the institution offering the course. However, a typical structure for the course may include the following:
- Introduction to Chemical Kinetics: This section introduces students to the fundamental concepts of chemical kinetics, including reaction rates, rate laws, and reaction order. It also covers the factors that influence reaction rates, such as temperature, concentration, and catalysts.
- Reaction Mechanisms: This section delves deeper into chemical kinetics by exploring the mechanisms behind chemical reactions. It covers topics such as elementary reactions, reaction intermediates, and reaction pathways.
- Catalysis: This section focuses on the role of catalysts in chemical reactions. It covers topics such as homogeneous and heterogeneous catalysis, enzyme catalysis, and the design of catalysts for specific reactions.
- Laboratory Experiments: Laboratory experiments are an important component of any chemical kinetics course. They allow students to apply the concepts they have learned in the classroom to real-world scenarios. Laboratory experiments may include topics such as reaction rate measurement, determination of rate laws, and investigation of reaction mechanisms.
- Applications of Chemical Kinetics: This section explores the practical applications of chemical kinetics in various fields, including materials science, energy, environmental engineering, and industrial processes.
- Problem-Solving and Analysis: Throughout the course, students are encouraged to develop their problem-solving and analytical skills. They are required to solve numerical problems related to chemical kinetics and apply their knowledge to real-world scenarios.
- Review and Assessment: At the end of the course, students will typically review the material covered and take assessments to demonstrate their understanding of the subject.
The structure of the JEE (Main+Advance) integrated course in chemical kinetics is designed to provide students with a comprehensive understanding of the subject, emphasizing the importance of understanding the fundamental concepts of chemical kinetics, their practical applications, and the development of problem-solving and analytical skills.
Application of JEE (Main+Advance) Integrated Course Chemical Kinetics
The application of JEE (Main+Advance) integrated course in chemical kinetics is diverse and wide-ranging, as chemical kinetics is a fundamental area of study that is applied in many fields. Some examples of the application of chemical kinetics include:
- Environmental Engineering: Chemical kinetics is essential in designing and optimizing environmental control systems that reduce air and water pollution. By understanding the rates and mechanisms of chemical reactions, engineers can develop effective strategies to control and mitigate the impact of pollutants.
- Materials Science: Chemical kinetics plays a crucial role in the development of new materials, such as polymers, composites, and ceramics. Understanding the rates and mechanisms of chemical reactions is critical in optimizing the synthesis and processing of materials.
- Energy: Chemical kinetics is used in the design and optimization of energy conversion processes, such as combustion, fuel cells, and batteries. By understanding the rates and mechanisms of chemical reactions, engineers can develop more efficient and sustainable energy systems.
- Pharmaceuticals: Chemical kinetics is used in the development of pharmaceuticals and drug delivery systems. Understanding the kinetics of drug release and metabolism is essential in optimizing drug efficacy and minimizing side effects.
- Industrial Processes: Chemical kinetics is used in the design and optimization of chemical processes in various industries, including petrochemicals, food and beverage, and manufacturing. By understanding the rates and mechanisms of chemical reactions, engineers can optimize the efficiency and productivity of industrial processes.
Overall, the application of JEE (Main+Advance) integrated course in chemical kinetics is crucial in many fields, as it provides a fundamental understanding of the rates and mechanisms of chemical reactions. This understanding is essential in developing new technologies, optimizing existing processes, and addressing many of the world’s most pressing challenges, such as environmental pollution and sustainable energy.
Nomenclature of JEE (Main+Advance) Integrated Course Chemical Kinetics
The nomenclature of JEE (Main+Advance) integrated course in chemical kinetics can vary depending on the institution offering the course. However, some common nomenclature used in the course includes:
- Reaction Rates: The rate at which a chemical reaction occurs, expressed in terms of the change in concentration of reactants or products per unit time.
- Rate Laws: The mathematical relationship between the reaction rate and the concentrations of reactants and catalysts.
- Reaction Order: The exponent of the concentration term in the rate law expression for a particular reactant.
- Activation Energy: The minimum amount of energy required for a chemical reaction to occur.
- Reaction Mechanisms: The series of elementary reactions that make up the overall chemical reaction.
- Reaction Intermediates: The unstable species that are formed during the course of a chemical reaction, but are not the reactants or products.
- Catalysts: Substances that increase the rate of a chemical reaction without being consumed in the process.
- Enzymes: Biological catalysts that are highly specific to particular reactions.
- Half-Life: The time required for the concentration of a reactant to decrease to half of its initial value.
- Kinetic Isotope Effect: The difference in reaction rate between isotopes of the same element due to their different masses.
These terms and concepts are essential in understanding the fundamental principles of chemical kinetics and its practical applications. By mastering the nomenclature of JEE (Main+Advance) integrated course in chemical kinetics, students can effectively communicate and apply their knowledge in a variety of fields.
Career Opportunities of JEE (Main+Advance) Integrated Course Chemical Kinetics
The career opportunities for students who have completed JEE (Main+Advance) integrated course in chemical kinetics are diverse and wide-ranging. Some of the career opportunities in this field include:
- Chemical Engineer: Chemical engineers use their knowledge of chemical kinetics to design and optimize chemical processes and equipment used in various industries, including pharmaceuticals, petrochemicals, and food and beverage.
- Environmental Engineer: Environmental engineers use chemical kinetics to design and implement systems that mitigate the impact of pollutants on the environment, including air and water pollution control systems.
- Materials Scientist: Materials scientists use chemical kinetics to develop and optimize the synthesis and processing of new materials, such as polymers, composites, and ceramics.
- Energy Engineer: Energy engineers use their knowledge of chemical kinetics to design and optimize energy conversion processes, such as combustion, fuel cells, and batteries.
- Pharmaceutical Scientist: Pharmaceutical scientists use chemical kinetics to develop and optimize drug delivery systems and pharmaceuticals.
- Research Scientist: Research scientists use chemical kinetics to study the rates and mechanisms of chemical reactions in various fields, including environmental science, materials science, and chemistry.
- Professor/Lecturer: Students who have completed JEE (Main+Advance) integrated course in chemical kinetics can pursue an academic career as a professor or lecturer in various fields, including chemical engineering, environmental science, materials science, and chemistry.
Overall, the career opportunities for students who have completed JEE (Main+Advance) integrated course in chemical kinetics are numerous and diverse, with opportunities in various industries and fields. The demand for professionals with a strong background in chemical kinetics is increasing, as this knowledge is essential in addressing many of the world’s most pressing challenges, such as environmental pollution, sustainable energy, and developing new materials and pharmaceuticals.
