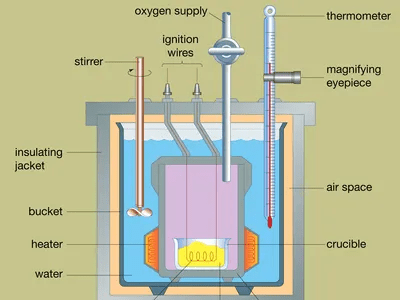
Calorimetry is the scientific measurement of heat transfer in a physical or chemical process. It involves the determination of the amount of heat absorbed or released by a substance or system during a physical or chemical change. Calorimetry is an important tool in various fields of science, including chemistry, physics, and engineering.
The basic principle of calorimetry is based on the conservation of energy, which states that energy can neither be created nor destroyed, only transformed from one form to another. In a calorimetric experiment, the heat absorbed or released by a substance is measured by monitoring the temperature change of a known mass of a calorimeter, a device designed to insulate and contain the reacting substances.
There are two types of calorimetry: constant-pressure calorimetry and constant-volume calorimetry. Constant-pressure calorimetry involves measuring the heat exchange between the reacting substances and their surroundings at a constant pressure, while constant-volume calorimetry measures the heat exchange at a constant volume.
Calorimetry has many practical applications, including the determination of the heat capacity and enthalpy of a substance, the measurement of the energy content of foods, and the analysis of chemical reactions.
What is Required Calorimetry
Required calorimetry is a type of calorimetry that is used to determine the amount of heat required to achieve a specific temperature change in a substance or system. This technique is often used in engineering and materials science to determine the thermal properties of materials, such as their specific heat capacity, thermal conductivity, and heat transfer coefficients.
The basic principle of required calorimetry involves measuring the heat required to change the temperature of a substance or system by a certain amount. This can be done using a calorimeter, which is a device that is designed to isolate the sample being tested from the surrounding environment and measure the amount of heat transferred between the sample and the calorimeter.
To perform required calorimetry, the sample is placed in the calorimeter and the initial temperature is recorded. A known amount of heat is then applied to the sample, and the temperature change is measured over time. The amount of heat required to achieve the desired temperature change can then be calculated based on the heat capacity of the sample and the calorimeter.
Required calorimetry can be used to determine a wide range of thermal properties, including the specific heat capacity of a material, which is a measure of the amount of heat required to raise the temperature of a unit mass of the material by one degree Celsius. This information can be useful in designing and optimizing thermal management systems, such as those used in electronic devices and power generation facilities.
When is Required Calorimetry
Required calorimetry is used in a variety of applications where it is important to measure the amount of heat required to achieve a specific temperature change in a substance or system. Here are a few examples:
- Materials science: Required calorimetry is commonly used in materials science to measure the specific heat capacity, thermal conductivity, and heat transfer coefficients of materials. This information is used in the design and optimization of thermal management systems, such as those used in electronic devices, power generation facilities, and building insulation.
- Chemical engineering: Required calorimetry is used in chemical engineering to measure the amount of heat required or released during chemical reactions. This information is used in the design and optimization of chemical processes, such as those used in the production of pharmaceuticals, fuels, and plastics.
- Food science: Required calorimetry is used in food science to measure the energy content of foods. This information is important for dietary and nutritional studies, as well as for food labeling and quality control.
- Environmental science: Required calorimetry is used in environmental science to measure the amount of heat required to raise or lower the temperature of bodies of water or soil. This information is used to study the effects of climate change and to monitor the thermal impacts of industrial activities on natural ecosystems.
Overall, required calorimetry is a versatile technique that has a wide range of applications in various fields of science and engineering.
Where is Required Calorimetry
Required calorimetry can be performed in a laboratory or industrial setting using specialized equipment called calorimeters. There are various types of calorimeters available, each with its own advantages and disadvantages depending on the application.
Laboratory-based required calorimetry is typically performed using one of the following types of calorimeters:
- Bomb calorimeter: A bomb calorimeter is a type of constant volume calorimeter that is used to measure the heat of combustion of a substance. The sample is placed inside a high-pressure oxygen bomb and ignited, and the heat released during the combustion process is measured.
- Differential scanning calorimeter (DSC): A DSC is a type of differential calorimeter that is used to measure the heat flow associated with thermal transitions in a sample, such as melting or crystallization. The sample is heated or cooled while the heat flow is continuously monitored, allowing for the determination of the sample’s specific heat capacity.
- Isothermal calorimeter: An isothermal calorimeter is a type of constant temperature calorimeter that is used to measure the heat flow associated with a chemical reaction. The sample and reagents are mixed inside the calorimeter, and the heat released or absorbed during the reaction is measured.
Industrial-based required calorimetry is typically performed using specialized equipment that is designed to measure the thermal properties of large-scale processes, such as chemical reactors or power generation facilities. These systems often use flow calorimeters or heat exchangers to measure the amount of heat required or released during a process.
Overall, required calorimetry can be performed in a variety of settings, from laboratory-based research to large-scale industrial processes, depending on the application.
How is Required Calorimetry
Required calorimetry involves measuring the amount of heat required to achieve a specific temperature change in a substance or system. The following is a general overview of how required calorimetry is typically performed:
- Sample preparation: The sample is prepared according to the requirements of the specific calorimetry technique being used. For example, in the case of DSC, the sample is typically ground to a fine powder and placed in a small aluminum pan.
- Calorimeter setup: The calorimeter is set up according to the requirements of the specific calorimetry technique being used. This may involve filling the calorimeter with a specific type of fluid, such as water, and ensuring that the sample is isolated from the surrounding environment.
- Baseline measurement: A baseline measurement is taken to determine the initial temperature of the sample and the calorimeter. This may involve allowing the system to equilibrate at a specific temperature for a set period of time.
- Heat application: A known amount of heat is applied to the sample, either through direct heating or through the addition of a reagent, depending on the specific calorimetry technique being used. The heat flow associated with the process is continuously monitored.
- Data analysis: The data collected during the calorimetry experiment is analyzed to determine the amount of heat required to achieve the desired temperature change. This may involve calculating the specific heat capacity of the sample, the enthalpy of reaction, or other thermal properties.
Overall, required calorimetry involves careful preparation of the sample and calorimeter, precise measurement of temperature and heat flow, and accurate data analysis. The specific techniques used may vary depending on the application and the equipment available.
Structures of Calorimetry
Calorimetry is a technique for measuring the heat flow associated with a physical or chemical process. There are several types of calorimeters, each with its own unique structure and operating principles. Here are a few examples:
- Bomb calorimeter: A bomb calorimeter is a type of constant volume calorimeter used to measure the heat of combustion of a substance. The calorimeter consists of a strong metal container (the bomb) that is filled with oxygen and the sample to be combusted. The bomb is then ignited, and the heat released during combustion is absorbed by the calorimeter’s water jacket. The temperature change of the water is measured to determine the heat of combustion.
- Differential scanning calorimeter (DSC): A DSC is a type of differential calorimeter that measures the heat flow associated with thermal transitions in a sample. The DSC typically consists of two sample cells, one containing the sample and the other containing a reference material. Both cells are subjected to a controlled temperature ramp, and the heat flow difference between the two cells is measured as a function of temperature.
- Isothermal calorimeter: An isothermal calorimeter is a type of constant temperature calorimeter used to measure the heat flow associated with a chemical reaction. The calorimeter typically consists of a reaction vessel surrounded by a water jacket that maintains a constant temperature. The sample and reagents are mixed inside the vessel, and the heat released or absorbed during the reaction is measured by the calorimeter’s temperature sensor.
- Flow calorimeter: A flow calorimeter is a type of calorimeter used to measure the heat flow associated with a flowing fluid. The calorimeter consists of a flow cell that contains a temperature sensor and a heating element. As the fluid flows through the cell, the heat flow is measured by monitoring the temperature difference across the cell.
Overall, the structures of calorimeters vary depending on the specific application and the type of calorimetry technique being used. However, all calorimeters share the common goal of accurately measuring the heat flow associated with a process.
Case Study on Calorimetry
Here is an example of a case study involving the use of calorimetry:
Case Study: Measuring the Specific Heat Capacity of a Metal
A laboratory was tasked with measuring the specific heat capacity of a metal sample. The sample was cylindrical in shape and had a mass of 50 grams. The laboratory used a differential scanning calorimeter (DSC) to perform the measurement.
The laboratory set up the DSC by placing the metal sample in a small aluminum pan, which was then sealed and placed in the DSC cell. A reference pan containing an empty aluminum pan was also placed in the DSC cell for comparison. Both pans were subjected to a temperature ramp from 25°C to 300°C at a rate of 10°C per minute.
During the temperature ramp, the laboratory observed a small exothermic peak in the heat flow curve around 100°C. This peak was attributed to the transformation of the metal from its alpha phase to its beta phase.
Using the heat flow data collected during the DSC experiment, the laboratory was able to calculate the specific heat capacity of the metal sample. The specific heat capacity was determined to be 0.47 J/g°C.
Based on the specific heat capacity measurement, the laboratory was able to identify the metal as copper, which has a specific heat capacity of approximately 0.39 J/g°C. The higher specific heat capacity measurement for the metal sample indicated that it was likely an alloy containing copper as one of its components.
This case study highlights how calorimetry, specifically DSC, can be used to measure the specific heat capacity of a material. The use of DSC allowed for precise temperature control and accurate measurement of the heat flow associated with thermal transitions in the sample, leading to a reliable measurement of its specific heat capacity.
White paper on Calorimetry
Introduction:
Calorimetry is the study of heat transfer and the measurement of the amount of heat released or absorbed during a chemical reaction, physical change or biological process. It is an essential technique used in many scientific disciplines, including chemistry, physics, and biology. Calorimetry is a very useful tool for understanding the energetics of a process, and it can provide insight into the thermodynamic properties of substances.
Principles of Calorimetry:
Calorimetry is based on the principle of conservation of energy, which states that energy cannot be created or destroyed, only transformed from one form to another. In a calorimetric experiment, the heat produced or absorbed by a process is measured and related to the change in energy of the system. This change in energy is quantified as the enthalpy change (ΔH) of the system.
Types of Calorimetry:
There are two main types of calorimetry: constant-pressure calorimetry and constant-volume calorimetry. In constant-pressure calorimetry, the heat is exchanged between the system and the surroundings at a constant pressure, usually atmospheric pressure. In this type of calorimetry, the heat absorbed or released by the system is measured as the change in enthalpy (ΔH) of the reaction. In constant-volume calorimetry, the heat is exchanged between the system and the surroundings at a constant volume. In this type of calorimetry, the heat absorbed or released by the system is measured as the change in internal energy (ΔU) of the system.
Applications of Calorimetry:
Calorimetry has many applications in various scientific fields. In chemistry, calorimetry is used to study the energetics of chemical reactions, including reaction enthalpies, heats of formation, and bond energies. It is also used to study phase transitions, such as melting and boiling points, and to determine the specific heat capacity of substances. In physics, calorimetry is used to study the properties of materials, such as their thermal conductivity and heat capacity. In biology, calorimetry is used to study metabolic processes, including respiration and fermentation, and to determine the caloric content of food.
Calorimetry Instruments:
Calorimetry instruments can be divided into two main categories: direct calorimeters and indirect calorimeters. Direct calorimeters measure the heat released or absorbed by a system directly, while indirect calorimeters measure the heat indirectly by measuring other properties of the system, such as the temperature, pressure, or volume.
Conclusion:
Calorimetry is an essential technique used in many scientific fields to study the energetics of chemical and physical processes. It is based on the principle of conservation of energy and provides valuable information about the thermodynamic properties of substances. Calorimetry instruments can be direct or indirect, and there are two main types of calorimetry: constant-pressure calorimetry and constant-volume calorimetry. Calorimetry is a powerful tool that has many applications in chemistry, physics, and biology, and it will continue to be an essential technique in scientific research.
