General principles and processes of isolations of elements
The general principles and processes of isolation of elements involve various techniques and methods used to extract and obtain pure elements from their ores or sources. Here are the fundamental principles and processes commonly employed:
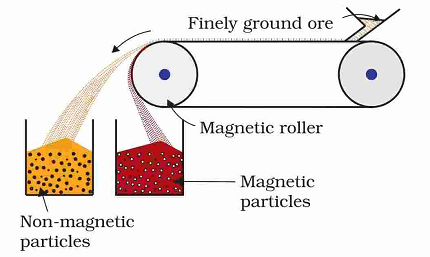
- Concentration of Ores: Most elements exist in the form of compounds in nature, commonly as ores. The concentration of ores involves the removal of impurities and gangue (unwanted materials) to obtain a concentrated form of the ore. Techniques such as gravity separation, froth flotation, magnetic separation, and leaching are used for this purpose.
- Reduction: Once the ore is concentrated, the next step is to extract the metal from the ore. Reduction is a process that involves the removal of oxygen or other elements from the compound to obtain the pure metal. Reduction can be achieved through various methods, including chemical reduction (roasting, calcination) and electrolytic reduction.
- Pyrometallurgy: Pyrometallurgical processes involve high-temperature operations to extract metals. These processes typically involve the use of heat to carry out smelting, roasting, or refining operations. Smelting involves the melting and separation of metal from its ore, while roasting and refining processes aim to remove impurities and obtain a purer form of the metal.
- Hydrometallurgy: Hydrometallurgical processes involve the use of aqueous solutions for the extraction of metals. Leaching is a common technique used in hydrometallurgy, where the ore is treated with a suitable solvent to selectively dissolve the metal into solution. The metal can then be recovered from the solution through precipitation or other methods.
- Electrometallurgy: Electrometallurgical processes utilize electrolysis to extract metals. In this process, the ore is dissolved in a suitable electrolyte, and an electric current is passed through the solution. The metal ions migrate towards the electrode of opposite charge and get reduced, leading to the deposition of pure metal on the electrode.
- Refining: Refining processes are employed to purify the extracted metal further. This involves removing impurities like non-metallic elements, other metals, or even isotopes of the same element. Techniques such as electrolytic refining, zone refining, and distillation are commonly used for this purpose.
- Other Techniques: Some elements require specific techniques for their isolation. For example, the production of highly reactive metals like sodium and potassium involves the electrolysis of their fused chlorides. The extraction of elements like carbon and silicon involves various reduction processes using reducing agents like coke or electric arc furnaces.
These general principles and processes of isolating elements form the basis for extracting metals and obtaining them in a pure form. The specific techniques employed may vary depending on the properties of the element, its abundance, and the nature of the ore or source material.
The syllabus for the “General Principles and Processes of Isolation of Elements” in the AIIMS (All India Institute of Medical Sciences) chemistry course may vary depending on the specific requirements of the institute. However, I can provide you with a general outline of the topics that are typically covered in this subject. Keep in mind that the syllabus may be subject to change, so it’s always a good idea to consult the official AIIMS syllabus or contact the institute directly for the most up-to-date information. Here’s an overview of the common topics covered in the syllabus:
- Occurrence of Metals: Sources of metals, minerals, ores, and gangue, general methods of extraction of metals.
- Concentration of Ores: Gravity separation, froth floatation method, magnetic separation, and leaching.
- Extraction of Crude Metal from Concentrated Ore: Principles and procedures of reduction by chemical methods (like roasting, calcination, etc.), and electrolytic reduction.
- Thermodynamics and Electrochemistry: Ellingham diagrams, Gibbs energy change, feasibility of reduction reactions, electrochemical principles involved in the extraction of metals.
- Principles of Metallurgical Operations: Roasting, calcination, smelting, refining, and alloying.
- Extraction of Some Important Metals: Aluminum, copper, iron, and zinc. Principles and processes involved in their extraction, purification, and uses.
- Principles of Hydrogen: Position of hydrogen in the periodic table, isotopes of hydrogen, and preparation, properties, and uses of hydrogen.
- Principles of Noble Gases: Isolation, physical and chemical properties, and uses of noble gases.
- Principles of Halogens: Isolation, physical and chemical properties, and uses of halogens.
- Principles of Oxygen and Sulfur: Isolation, physical and chemical properties, and uses of oxygen and sulfur.
- Principles of Nitrogen and Phosphorus: Isolation, physical and chemical properties, and uses of nitrogen and phosphorus.
- Principles of Carbon and Silicon: Isolation, physical and chemical properties, and uses of carbon and silicon.
- Environmental Chemistry: Environmental pollution, greenhouse effect, acid rain, ozone depletion, and water pollution.
It’s important to note that the syllabus may include additional or more specific topics related to the general principles and processes of isolating elements. It’s always recommended to refer to the official AIIMS syllabus or course curriculum for precise details.
What is Required Chemistry syllabus General principles and processes of isolations of elements
The required chemistry syllabus for the topic “General Principles and Processes of Isolation of Elements” may vary depending on the specific educational institution or examination board. However, I can provide you with a general outline of the topics commonly included in this subject. Here are the key components that are typically covered in the syllabus:
- Occurrence of Metals: Sources of metals, minerals, ores, and gangue. Classification of ores based on the nature of their occurrence (oxidation, sulfides, carbonates, etc.). General methods of extraction of metals.
- Concentration of Ores: Principles and methods of concentration of ores, including gravity separation, magnetic separation, froth flotation, and leaching.
- Extraction of Crude Metal from Concentrated Ore: Principles and procedures of reduction by chemical methods, such as roasting, calcination, and smelting. Introduction to electrolytic reduction and its application in metal extraction.
- Thermodynamics and Electrochemistry: Understanding the principles of thermodynamics and electrochemistry in relation to the extraction of metals. Application of Gibbs energy change and Ellingham diagrams to determine the feasibility of reduction reactions.
- Principles of Metallurgical Operations: Overview of important metallurgical operations, including roasting, calcination, smelting, refining, and alloying.
- Extraction of Some Important Metals: Detailed study of the extraction, purification, and uses of specific metals like iron, copper, aluminum, and zinc.
- Principles of Hydrogen: Position of hydrogen in the periodic table, isotopes of hydrogen, preparation, properties, and uses of hydrogen.
- Principles of Noble Gases: Isolation, physical and chemical properties, and uses of noble gases.
- Principles of Halogens: Isolation, physical and chemical properties, and uses of halogens.
- Principles of Oxygen and Sulfur: Isolation, physical and chemical properties, and uses of oxygen and sulfur.
- Principles of Nitrogen and Phosphorus: Isolation, physical and chemical properties, and uses of nitrogen and phosphorus.
- Principles of Carbon and Silicon: Isolation, physical and chemical properties, and uses of carbon and silicon.
- Environmental Chemistry: Introduction to environmental pollution, greenhouse effect, acid rain, ozone depletion, and water pollution.
Please note that this is a general outline, and the specific syllabus may include additional topics or subtopics related to the general principles and processes of isolating elements. It is recommended to refer to the official syllabus provided by the educational institution or examination board for precise details and comprehensive coverage of the subject.
When is Required Chemistry syllabus General principles and processes of isolations of elements
The specific timing of when the “General Principles and Processes of Isolation of Elements” topic is covered in the chemistry syllabus can vary depending on the educational institution, curriculum, or examination board. Typically, this topic is included in the syllabus of higher secondary education (grades 11 and 12) or undergraduate-level chemistry courses.
In high school chemistry curricula, the topic of isolation of elements is often covered in the section dedicated to “Metallurgy” or “Extraction of Metals.” It is usually taught after foundational topics such as atomic structure, periodicity, and chemical bonding.
For undergraduate-level chemistry courses, the specific timing of when the topic is covered may vary depending on the curriculum structure and the institution’s sequencing of subjects. Generally, it is covered in the early stages of the course, as it provides fundamental knowledge and understanding of the principles involved in the extraction of elements.
It’s important to note that the sequencing of topics may differ between educational institutions, and the syllabus may be subject to change. It is always recommended to refer to the official syllabus or curriculum provided by the respective educational institution or examination board for the most accurate and up-to-date information regarding the timing and coverage of the “General Principles and Processes of Isolation of Elements” topic.
Where is Required Chemistry syllabus General principles and processes of isolations of elements
The “General Principles and Processes of Isolation of Elements” topic is typically included in the chemistry syllabus of higher secondary education (grades 11 and 12) or undergraduate-level chemistry courses. The exact location of this topic within the syllabus may vary depending on the specific curriculum or educational institution. However, it is commonly found in the section dedicated to “Metallurgy” or “Extraction of Metals.”
In high school chemistry curricula, this topic is usually covered after foundational topics such as atomic structure, periodicity, chemical bonding, and basic concepts of stoichiometry. It is often part of the broader section on inorganic chemistry.
For undergraduate-level chemistry courses, the syllabus structure may vary depending on the institution and the specific degree program. The topic of isolation of elements is typically covered early in the course, as it provides essential knowledge and understanding of the principles involved in extracting elements from their ores or sources. It may be included in a dedicated section on inorganic chemistry or as part of a broader course on general chemistry.
It’s important to note that the organization and placement of topics within a syllabus may differ between educational institutions or examination boards. Therefore, it is recommended to refer to the official syllabus or curriculum provided by the respective educational institution or examination board to determine the specific location of the “General Principles and Processes of Isolation of Elements” topic within the chemistry syllabus.
How is Required Chemistry syllabus General principles and processes of isolations of elements
The “General Principles and Processes of Isolation of Elements” topic in the chemistry syllabus is typically taught using a combination of theoretical concepts, practical demonstrations, and laboratory experiments. Here’s a general overview of how this topic is covered:
- Theoretical Concepts: The topic begins with an introduction to the occurrence of metals in nature and the various types of ores and minerals. Students learn about the classification of ores based on their composition and the techniques used for their extraction. The principles underlying the extraction of metals, such as reduction reactions and thermodynamics, are also covered.
- Extraction Methods: The syllabus delves into the different methods used to concentrate ores and remove impurities. Students learn about techniques like gravity separation, froth flotation, magnetic separation, and leaching, and understand the principles behind each method.
- Reduction Processes: The syllabus covers the various reduction processes used to extract metals from their ores. Students learn about chemical reduction methods, including roasting and calcination, which involve the application of heat to convert ores into their respective oxides. The principles of smelting and electrolytic reduction are also taught, with a focus on the principles and apparatus involved.
- Metallurgical Operations: The syllabus includes an overview of important metallurgical operations, such as roasting, calcination, smelting, refining, and alloying. Students gain an understanding of the purpose and procedure of these operations, as well as the equipment used.
- Extraction of Specific Metals: The syllabus covers the extraction, purification, and uses of specific metals. Common metals like iron, copper, aluminum, and zinc are typically studied in detail, including their extraction processes, purification methods, and industrial applications.
- Environmental Aspects: The syllabus may also include a section on environmental chemistry, focusing on the environmental impact of metal extraction and refining processes. Topics such as environmental pollution, acid rain, water pollution, and the importance of sustainable practices in metallurgy may be covered.
- Practical Demonstrations and Experiments: The syllabus often includes practical sessions where students observe and perform experiments related to the extraction and purification of metals. This hands-on experience helps reinforce theoretical concepts and allows students to gain practical skills in laboratory techniques.
It’s important to note that the actual content and depth of coverage may vary based on the specific educational institution, examination board, or course level. It is recommended to refer to the official syllabus or curriculum provided by the respective educational institution or examination board for precise details on the topics covered and the specific teaching methods employed.
Structures of Chemistry syllabus General principles and processes of isolations of elements
While I can provide you with a general outline of the topics covered in the “General Principles and Processes of Isolation of Elements” section of the chemistry syllabus, I do not have access to specific institutional syllabi or examination boards. The actual structure of the syllabus may vary depending on the educational institution, examination board, or curriculum followed. However, here is a typical structure of the topics covered in the syllabus:
- Introduction
- Occurrence of metals in nature
- Minerals, ores, and gangue
- Sources of metals
- Concentration of Ores
- Principles and methods of concentration
- Gravity separation
- Froth flotation
- Magnetic separation
- Leaching
- Extraction of Metals
- Reduction of ores
- Chemical reduction methods (roasting, calcination)
- Smelting and its principles
- Electrolytic reduction
- Thermodynamics and Electrochemistry
- Gibbs energy change and its application in metal extraction
- Ellingham diagrams
- Feasibility of reduction reactions
- Electrochemical principles in metal extraction
- Metallurgical Operations
- Roasting
- Calcination
- Smelting
- Refining
- Alloying
- Extraction of Specific Metals
- Aluminum
- Copper
- Iron
- Zinc
- Other selected metals
- Environmental Chemistry
- Environmental pollution
- Greenhouse effect
- Acid rain
- Ozone depletion
- Water pollution
Please note that the above structure is a general outline and may not include all the subtopics or specific details. The actual syllabus structure may vary based on the institution or examination board. It’s recommended to consult the official syllabus provided by the respective educational institution or examination board for the most accurate and up-to-date information on the specific structure of the “General Principles and Processes of Isolation of Elements” topic in the chemistry syllabus.
Case Study on Chemistry syllabus General principles and processes of isolations of elements
Sure! Here’s a case study that illustrates the application of general principles and processes of isolation of elements:
Case Study: Extraction of Copper from Copper Ore
Background: A mining company has discovered a copper ore deposit in a remote location. They want to extract copper from the ore and process it into a usable form for various applications.
Objective: To design a process for the extraction of copper from the copper ore using the general principles and processes of isolation of elements.
Solution:
- Ore Concentration: The copper ore needs to be concentrated to remove impurities and gangue minerals. Gravity separation, froth flotation, and magnetic separation can be used to achieve ore concentration. In this case, froth flotation is selected as the preferred method. The finely crushed copper ore is mixed with water and chemicals, such as collectors and frothers. Air is then blown through the mixture to create bubbles. The copper minerals attach to the bubbles and rise to the surface, forming a froth layer, while the gangue particles sink. The froth containing copper is collected for further processing.
- Smelting: Smelting is the process of extracting the metal from the concentrated ore. In the case of copper, the froth obtained from the flotation process contains copper sulfide minerals. The froth is dried and heated in a furnace to undergo smelting. The copper sulfide minerals react with oxygen in the air to form copper(I) oxide (Cu2O), which further reacts with unreacted copper sulfide to form copper metal (Cu). The impurities and gangue materials combine to form slag, which is separated from the molten copper.
- Electrorefining: The obtained crude copper from the smelting process contains impurities like iron, sulfur, and other metals. Electrorefining is used to purify the crude copper and obtain a high-purity form suitable for various applications. In this process, the crude copper acts as an anode, and a pure copper cathode is used. Both are immersed in an electrolyte solution containing copper sulfate. When an electric current is passed through the electrolyte, copper ions from the anode are oxidized and transferred to the cathode, resulting in the deposition of pure copper on the cathode. The impurities settle at the bottom of the electrolytic cell as anode slime.
- Further Processing: The high-purity copper obtained through electrorefining can be further processed into various forms, such as copper sheets, wires, or rods, depending on the specific applications required.
Conclusion: Through the application of general principles and processes of isolation of elements, the mining company successfully extracted copper from the ore deposit. The process involved ore concentration using froth flotation, followed by smelting to obtain copper metal, and electrorefining to purify the copper. The resulting high-purity copper can be utilized in various industrial applications.
Note: This case study provides a simplified overview of the extraction process and does not cover all the specific details and considerations involved in the actual industrial processes.
White paper on Chemistry syllabus General principles and processes of isolations of elements
Title: General Principles and Processes of Isolation of Elements: A Comprehensive White Paper
Abstract:
The isolation of elements from their natural sources is a fundamental process in chemistry and plays a vital role in various industries. This white paper provides an in-depth exploration of the general principles and processes involved in the isolation of elements. It covers topics such as ore concentration, reduction methods, thermodynamics, electrochemistry, metallurgical operations, and the extraction of specific metals. The paper also discusses the environmental aspects associated with the extraction processes. Through a comprehensive examination of these principles and processes, this white paper aims to enhance the understanding of researchers, students, and professionals in the field of chemistry.
Introduction
1.1 Importance of the Isolation of Elements
1.2 Overview of the General Principles and Processes
Occurrence of Metals and Sources
2.1 Sources of Metals in Nature
2.2 Minerals, Ores, and Gangue
2.3 Classification of Ores based on Occurrence
Concentration of Ores
3.1 Principles of Ore Concentration
3.2 Gravity Separation
3.3 Froth Flotation
3.4 Magnetic Separation
3.5 Leaching
Reduction Methods
4.1 Chemical Reduction Methods
4.1.1 Roasting
4.1.2 Calcination
4.2 Smelting and Principles
4.3 Electrolytic Reduction
Thermodynamics and Electrochemistry
5.1 Gibbs Energy Change and its Application
5.2 Ellingham Diagrams
5.3 Feasibility of Reduction Reactions
5.4 Electrochemical Principles in Metal Extraction
Metallurgical Operations
6.1 Roasting
6.2 Calcination
6.3 Smelting
6.4 Refining
6.5 Alloying
Extraction of Specific Metals
7.1 Aluminum
7.2 Copper
7.3 Iron
7.4 Zinc
7.5 Other Selected Metals
Environmental Aspects
8.1 Environmental Pollution
8.2 Greenhouse Effect
8.3 Acid Rain
8.4 Ozone Depletion
8.5 Water Pollution
Conclusion
9.1 Summary of General Principles and Processes
9.2 Significance of Isolation of Elements
9.3 Future Perspectives and Advances in the Field
References
This white paper provides a comprehensive overview of the general principles and processes involved in the isolation of elements. It serves as a valuable resource for researchers, educators, and professionals in the field of chemistry, offering a deeper understanding of the underlying concepts and techniques utilized in the extraction of metals from their ores or sources. By exploring the environmental aspects associated with these processes, this white paper also highlights the importance of sustainable practices in the field of element isolation.
