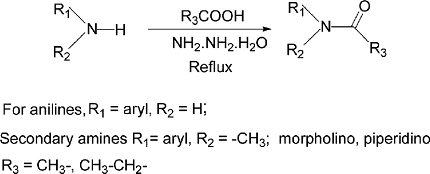
Acylation reactions are chemical reactions in which an acyl group (-COCH3) is added to a molecule. The acyl group can be derived from an acid chloride (RCOCl), an anhydride (RCOOR), or a carboxylic acid (RCOOH) with an activating agent such as DCC (dicyclohexylcarbodiimide) or SOCl2 (thionyl chloride).
One common type of acylation reaction is the Friedel-Crafts acylation, which involves the reaction of an aromatic compound with an acyl halide in the presence of a Lewis acid catalyst, typically aluminum chloride (AlCl3) or iron(III) chloride (FeCl3). This reaction is used to introduce an acyl group onto an aromatic ring and is an important method for the synthesis of pharmaceuticals, agrochemicals, and other organic compounds.
Another type of acylation reaction is the peptide bond formation in protein synthesis. This reaction involves the condensation of an amino acid with a carboxylic acid to form a peptide bond (-CO-NH-) and release a molecule of water. The reaction is catalyzed by ribosomes and is essential for the synthesis of proteins in living organisms.
Overall, acylation reactions are important in organic chemistry and have a wide range of applications in the synthesis of various compounds, including pharmaceuticals, natural products, and polymers.
What is Required Amines Acylation reactions
Acylation reactions can be used to introduce an acyl group (-COCH3) onto an amine group (-NH2) to form an amide (-CONH2). This reaction is commonly known as amide synthesis or acylation of amines. The reaction can be catalyzed by an acid catalyst such as HCl or H2SO4, or by a base catalyst such as pyridine or triethylamine.
The general reaction equation for the acylation of an amine is:
R-CO-X + R’NH2 → R-CO-NH-R’ + HX
where R and R’ represent alkyl or aryl groups and X represents a leaving group such as a halogen (Cl, Br) or a sulfonate ester (OTs, OMs). The leaving group X is often derived from an acylating agent such as an acid chloride (RCOCl) or an anhydride (RCOOCOR’).
The amide synthesis reaction can also be used to prepare primary, secondary, and tertiary amides. For example, a primary amide can be prepared by the reaction of an acid chloride with ammonia (NH3), while a secondary amide can be prepared by the reaction of an acid chloride with a primary amine. Tertiary amides can be prepared by the reaction of an acid chloride with a secondary amine.
Overall, the acylation of amines is an important reaction in organic synthesis, and amides are ubiquitous in nature and have many important applications in the pharmaceutical, chemical, and materials industries.
When is Required Amines Acylation reactions
The acylation of amines is a useful reaction in organic chemistry and is used in a wide range of applications. Here are some examples of when acylation of amines is required:
- Synthesis of amides: One of the main applications of acylation of amines is in the synthesis of amides. Amides are important functional groups in organic chemistry and are found in a variety of natural and synthetic compounds such as peptides, proteins, and polymers.
- Preparation of pharmaceuticals: The acylation of amines is commonly used in the synthesis of pharmaceuticals. For example, the synthesis of penicillin, a widely used antibiotic, involves the acylation of an amine group in a β-lactam ring with an acylating agent.
- Preparation of agrochemicals: The acylation of amines is also used in the synthesis of agrochemicals, such as herbicides and insecticides. For example, the synthesis of the herbicide glyphosate involves the acylation of an amine group with an acylating agent.
- Preparation of polymers: The acylation of amines is used in the preparation of polymers. For example, polyamides (nylon) are synthesized by the reaction of diamines with diacids or diacid chlorides.
Overall, the acylation of amines is an important reaction in organic chemistry and has many practical applications in the synthesis of a wide range of compounds, including pharmaceuticals, agrochemicals, and polymers.
Where is Required Amines Acylation reactions
The acylation of amines is a widely used reaction in organic chemistry and can be carried out in various settings, such as in academic research laboratories, industrial settings, and pharmaceutical companies. Here are some examples of where acylation of amines may be required:
- Academic research laboratories: The acylation of amines is a fundamental reaction in organic chemistry and is often taught and researched in academic laboratories. Researchers may use this reaction to synthesize new compounds, study reaction mechanisms, or develop new synthetic methodologies.
- Pharmaceutical industry: The acylation of amines is an important reaction in the pharmaceutical industry. It is used to synthesize a variety of drugs, such as antibiotics, antivirals, and antipsychotics. This reaction can be carried out on a large scale in pharmaceutical companies to produce drugs in commercial quantities.
- Agrochemical industry: The acylation of amines is also used in the agrochemical industry. It is used to synthesize herbicides, insecticides, and fungicides that are used to protect crops from pests and diseases.
- Polymer industry: The acylation of amines is used in the polymer industry to synthesize polymers such as polyamides (nylon). These polymers have a wide range of applications, such as in textiles, automotive components, and packaging materials.
Overall, the acylation of amines is a versatile reaction that can be carried out in various settings for different purposes. It is an important reaction in organic chemistry that has many practical applications in industries such as pharmaceuticals, agrochemicals, and polymers.
How is Required Amines Acylation reactions
The acylation of amines involves the reaction of an amine with an acylating agent to form an amide. The reaction can be catalyzed by either an acid catalyst or a base catalyst. Here are the general steps for the acylation of amines:
- Activation of the acylating agent: The acylating agent, such as an acid chloride or an anhydride, is first activated by a catalyst such as a Lewis acid or a tertiary amine. This makes the acylating agent more reactive towards nucleophilic attack by the amine.
- Nucleophilic attack: The amine attacks the activated acylating agent to form an intermediate, which is an acylated amine.
- Protonation: The intermediate is then protonated by either the acid catalyst or the amine itself to form an amide.
- Workup: The reaction mixture is usually quenched with a weak acid or base to neutralize any remaining catalyst, and the product is purified by standard methods such as crystallization or chromatography.
Here is an example reaction for the acylation of an amine using an acid chloride:
R-CO-Cl + R’NH2 → R-CO-NH-R’ + HCl
In this reaction, R and R’ are organic groups, and HCl is the protonated form of the acid chloride. The reaction is typically carried out in an organic solvent such as dichloromethane or chloroform.
Overall, the acylation of amines is a straightforward reaction that can be carried out using a variety of acylating agents and catalysts. The reaction mechanism is well understood, and the reaction conditions can be optimized for a specific reaction to achieve high yields and selectivity.
Structures of Amines Acylation reactions
The acylation of amines involves the reaction of an amine with an acylating agent, which typically results in the formation of an amide product. The general structure of an amide is R-CO-NH-R’, where R and R’ can be either alkyl or aryl groups. The structure of the amide product will depend on the specific acylating agent used and the conditions of the reaction.
Here are some examples of the structures of amides that can be formed via the acylation of amines:
- N,N-dimethylacetamide: This amide is formed by the reaction of dimethylamine with acetyl chloride. Its structure is CH3CON(CH3)2.
- Benzamide: This amide is formed by the reaction of aniline with benzoyl chloride. Its structure is PhCONH2, where Ph is the phenyl group.
- N-acetylglycine: This amide is formed by the reaction of glycine with acetic anhydride. Its structure is CH3CO-NH-CH2-COOH.
- N-methylbenzamide: This amide is formed by the reaction of methylamine with benzoic acid. Its structure is PhCONHCH3.
Overall, the specific structure of the amide product will depend on the specific amine and acylating agent used in the reaction. The acylation of amines is a versatile reaction that can be used to synthesize a wide range of amides with different structures and properties.
Case Study on Amines Acylation reactions
One example of the application of the acylation of amines is in the synthesis of the drug ibuprofen. Ibuprofen is a nonsteroidal anti-inflammatory drug (NSAID) that is used to treat pain, fever, and inflammation.
The synthesis of ibuprofen involves several steps, including the acylation of an amine to form an amide intermediate. Here is a brief overview of the synthesis:
- The starting material for the synthesis is the compound benzene, which is nitrated to form nitrobenzene.
- The nitro group on nitrobenzene is then reduced to an amine group, forming phenylamine (also known as aniline).
- Phenylamine is acylated with isobutyric anhydride in the presence of a base catalyst such as pyridine. This forms the amide intermediate, N-phenylacetyl isobutylamide.
- The amide intermediate is then reduced with lithium aluminum hydride to form the alcohol intermediate, which is then oxidized with a mild oxidizing agent to form ibuprofen.
The acylation of phenylamine is a key step in the synthesis of ibuprofen, as it forms the amide intermediate that is further modified to form the final product. The reaction is typically carried out in a solvent such as dichloromethane, and the yield of the reaction can be optimized by adjusting the reaction conditions.
Overall, the acylation of amines is an important reaction in the synthesis of many pharmaceuticals, including ibuprofen. The reaction is versatile and can be used to form a wide range of amides, which can then be further modified to form a variety of compounds.
White paper on Amines Acylation reactions
Introduction:
Amines are organic compounds that contain a nitrogen atom bonded to one or more alkyl or aryl groups. They are widely used in pharmaceuticals, agrochemicals, and materials science due to their diverse chemical properties. One important reaction that involves amines is acylation, which is the reaction of an amine with an acylating agent to form an amide. The acylation of amines is a versatile reaction that can be used to synthesize a wide range of compounds with different structures and properties.
Acylation Reactions:
The acylation of amines involves the reaction of an amine with an acylating agent, such as an acid chloride or an anhydride. The reaction can be catalyzed by either an acid catalyst or a base catalyst, depending on the specific reaction conditions. The general reaction mechanism involves the activation of the acylating agent, nucleophilic attack by the amine, protonation, and workup to purify the product. The resulting product is an amide, which can be further modified to form a variety of compounds.
Applications of Amine Acylation:
The acylation of amines has many important applications in the synthesis of pharmaceuticals, agrochemicals, and materials science. For example, the synthesis of ibuprofen involves the acylation of an amine to form an amide intermediate. This intermediate is then further modified to form the final product, which is a nonsteroidal anti-inflammatory drug. Other examples of compounds synthesized using the acylation of amines include amides, lactams, peptides, and polymers.
Advantages of Amine Acylation:
The acylation of amines has several advantages over other methods of synthesis. First, it is a relatively simple and straightforward reaction that can be carried out using a variety of acylating agents and catalysts. Second, it is a versatile reaction that can be used to synthesize a wide range of compounds with different structures and properties. Finally, it is a cost-effective method of synthesis that can be easily scaled up for commercial production.
Conclusion:
The acylation of amines is an important reaction in the synthesis of many pharmaceuticals, agrochemicals, and materials science. It is a versatile and cost-effective method of synthesis that can be easily optimized for specific reactions to achieve high yields and selectivity. Overall, the acylation of amines is an essential tool for chemists in a variety of fields, and its applications will continue to grow as new compounds are developed.
