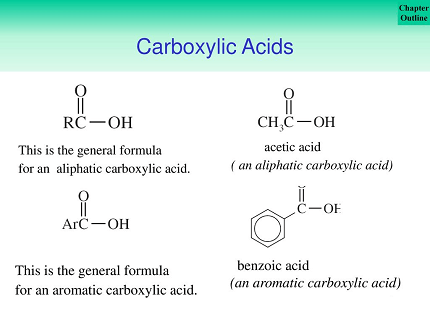
Aliphatic carboxylic acids are organic compounds that contain a carboxyl functional group (-COOH) attached to an aliphatic carbon chain. The carboxyl group is composed of a carbonyl group (C=O) and a hydroxyl group (OH). These compounds are commonly known as organic acids.
Aliphatic carboxylic acids can be classified into three main groups based on the length of their carbon chain:
- Short-chain carboxylic acids (up to 6 carbons), such as formic acid (HCOOH) and acetic acid (CH3COOH), which are commonly found in nature and are used in many industrial applications.
- Medium-chain carboxylic acids (6-12 carbons), such as caprylic acid (C7H15COOH) and lauric acid (C11H23COOH), which are used in the production of soaps, detergents, and cosmetics.
- Long-chain carboxylic acids (more than 12 carbons), such as stearic acid (C17H35COOH) and oleic acid (C18H33COOH), which are used in the production of candles, lubricants, and plastics.
Aliphatic carboxylic acids are important building blocks in organic chemistry and are widely used in various industries. They can also be found in nature, where they play important roles in metabolic processes and as components of lipids and fats.
History of Aliphatic carboxylic acids
The history of aliphatic carboxylic acids dates back to the early days of organic chemistry, when scientists first began to study the properties and reactions of organic compounds. One of the earliest known aliphatic carboxylic acids is acetic acid, which was discovered by the Roman scholar and naturalist, Pliny the Elder, in the 1st century AD.
However, it was not until the 18th and 19th centuries that significant progress was made in the study of aliphatic carboxylic acids. In 1779, the Swedish chemist Carl Wilhelm Scheele isolated acetic acid in a pure form by heating vinegar with sulfuric acid. This was a significant achievement, as it allowed scientists to study the properties of acetic acid and paved the way for further research into other carboxylic acids.
In the early 19th century, the French chemist Jean-Baptiste Dumas discovered the homologous series of carboxylic acids, which includes a series of compounds with similar chemical and physical properties but with increasing carbon chain length. This led to the discovery of many new aliphatic carboxylic acids, including formic acid, propionic acid, butyric acid, and many others.
During the 20th century, aliphatic carboxylic acids became increasingly important in industrial applications. They are used in the production of a wide range of products, including pharmaceuticals, plastics, textiles, and food additives. Today, the study of aliphatic carboxylic acids continues to be an important area of research in organic chemistry, with many new compounds and applications still being discovered.
Types of Aliphatic carboxylic acids
Aliphatic carboxylic acids can be classified into three main types based on the length of their carbon chain: short-chain, medium-chain, and long-chain.
- Short-chain carboxylic acids: These are carboxylic acids with up to six carbon atoms in their carbon chain. Examples of short-chain carboxylic acids include formic acid (HCOOH), acetic acid (CH3COOH), propionic acid (C2H5COOH), and butyric acid (C3H7COOH). These compounds are commonly found in nature and have many industrial applications.
- Medium-chain carboxylic acids: These are carboxylic acids with 6 to 12 carbon atoms in their carbon chain. Examples of medium-chain carboxylic acids include caproic acid (C5H11COOH), caprylic acid (C7H15COOH), and lauric acid (C11H23COOH). These compounds are used in the production of soaps, detergents, and cosmetics.
- Long-chain carboxylic acids: These are carboxylic acids with more than 12 carbon atoms in their carbon chain. Examples of long-chain carboxylic acids include stearic acid (C17H35COOH), oleic acid (C18H33COOH), and linoleic acid (C18H32O2). These compounds are used in the production of candles, lubricants, and plastics.
Aliphatic carboxylic acids can also be further classified based on the presence of functional groups or other structural features. For example, aromatic carboxylic acids contain an aromatic ring in addition to the carboxyl group, and unsaturated carboxylic acids contain one or more double or triple bonds in their carbon chain.
Structures of Aliphatic carboxylic acids
Aliphatic carboxylic acids have a common structure, characterized by a carboxyl functional group (-COOH) attached to an aliphatic carbon chain. The carboxyl group consists of a carbonyl group (C=O) and a hydroxyl group (-OH) attached to the same carbon atom.
The general formula for an aliphatic carboxylic acid is R-COOH, where R is the alkyl group or hydrogen attached to the carbonyl carbon. In this formula, the carbon atom of the carboxyl group is always numbered as carbon 1, and the carbon atoms of the alkyl chain are numbered consecutively starting from the carbon attached to the carboxyl group.
Here are some examples of the structures of aliphatic carboxylic acids:
- Methanoic acid (formic acid): HCOOHH-C=O | H
- Ethanoic acid (acetic acid): CH3COOHCH3-C=O | OH
- Propanoic acid: CH3CH2COOHCH3-CH2-C=O | OH
- Butanoic acid: CH3CH2CH2COOHCH3-CH2-CH2-C=O | OH
- Octanoic acid: CH3(CH2)6COOHCH3-CH2-CH2-CH2-CH2-CH2-CH2-C=O | OH
In all of these structures, the carboxyl group is always present at the end of the carbon chain, and the alkyl chain can vary in length and branching.
Nomenclature of Aliphatic carboxylic acids
The nomenclature of aliphatic carboxylic acids follows the rules of the International Union of Pure and Applied Chemistry (IUPAC). The name of the carboxylic acid is derived from the name of the corresponding alkane with the same number of carbon atoms, by replacing the suffix “-ane” with “-anoic acid”. Here are the general steps for naming aliphatic carboxylic acids:
- Identify the number of carbon atoms in the carboxylic acid’s carbon chain.
- Determine the name of the corresponding alkane with the same number of carbon atoms.
- Replace the suffix “-ane” with “-anoic acid” to obtain the name of the carboxylic acid.
For example:
- Methanoic acid: This is the simplest aliphatic carboxylic acid, with one carbon atom in its carbon chain. The corresponding alkane is methane, so the name of the carboxylic acid is derived by replacing “-ane” with “-anoic acid”. Therefore, the IUPAC name of methanoic acid is formic acid.
- Ethanoic acid: This is a carboxylic acid with two carbon atoms in its carbon chain. The corresponding alkane is ethane, so the name of the carboxylic acid is derived by replacing “-ane” with “-anoic acid”. Therefore, the IUPAC name of ethanoic acid is acetic acid.
- Propanoic acid: This is a carboxylic acid with three carbon atoms in its carbon chain. The corresponding alkane is propane, so the name of the carboxylic acid is derived by replacing “-ane” with “-anoic acid”. Therefore, the IUPAC name of propanoic acid is propionic acid.
Note that in cases where there are substituent groups attached to the carbon chain, the name of the carboxylic acid can become more complex, and a numbering system is used to indicate the location of the substituents on the carbon chain.
Production of Aliphatic carboxylic acids
Aliphatic carboxylic acids can be produced through various methods, including oxidation of primary alcohols, hydrocarbons, and aldehydes, as well as through the carboxylation of organometallic compounds. Here are some common methods for the production of aliphatic carboxylic acids:
- Oxidation of primary alcohols: Primary alcohols can be oxidized to form aldehydes, which can then be further oxidized to form carboxylic acids. The oxidation can be carried out using various oxidizing agents, such as potassium permanganate, chromic acid, and nitric acid.
- Oxidation of hydrocarbons: Hydrocarbons can be oxidized to form aldehydes, which can then be further oxidized to form carboxylic acids. The oxidation can be carried out using various oxidizing agents, such as potassium permanganate, chromic acid, and nitric acid.
- Carboxylation of organometallic compounds: Organometallic compounds, such as Grignard reagents, can be carboxylated to form carboxylic acids. The reaction involves the addition of carbon dioxide to the organometallic compound in the presence of a catalyst, such as copper or nickel.
- Fermentation: Carboxylic acids can also be produced through fermentation of sugars or other organic compounds. For example, acetic acid can be produced through the fermentation of ethanol by acetic acid bacteria.
- Halogenation and hydrolysis of alkyl halides: Alkyl halides can be halogenated to form carboxylic acid halides, which can then be hydrolyzed to form carboxylic acids. The hydrolysis can be carried out using water or a base, such as sodium hydroxide.
Overall, the production of aliphatic carboxylic acids depends on the specific starting materials and reaction conditions used.
Case Study on Aliphatic carboxylic acids
One interesting case study on aliphatic carboxylic acids is their use in the production of biodegradable polymers. Poly(lactic acid) (PLA) is a biodegradable polymer that is produced from lactic acid, an aliphatic carboxylic acid. PLA is an alternative to traditional petroleum-based plastics, which can take hundreds of years to biodegrade and contribute to environmental pollution.
PLA is produced through the polymerization of lactic acid, which can be obtained through fermentation of corn or other plant-based materials. The lactic acid is then purified and polymerized to form PLA. The resulting polymer has properties similar to traditional plastics, including transparency, toughness, and durability, but it is biodegradable and compostable, making it a more sustainable alternative.
The production of PLA has several advantages over traditional petroleum-based plastics. First, it is derived from renewable resources, such as corn or other plant-based materials, which reduces the dependence on fossil fuels. Second, PLA is biodegradable and compostable, which reduces environmental pollution and waste. Third, PLA has similar properties to traditional plastics, making it a viable alternative for a range of applications, including packaging, textiles, and medical devices.
However, there are also some challenges associated with the production of PLA. For example, the production of lactic acid requires large amounts of water and energy, and the fermentation process can be sensitive to impurities and pH changes. Additionally, the production of PLA is currently more expensive than traditional plastics, which limits its widespread adoption.
Overall, the use of aliphatic carboxylic acids, such as lactic acid, in the production of biodegradable polymers has the potential to reduce environmental pollution and waste, and to promote a more sustainable future.
White paper on Aliphatic carboxylic acids
Introduction:
Aliphatic carboxylic acids are organic compounds that have a carboxyl group (-COOH) attached to an aliphatic carbon chain. They are widely used in various industries, including food, pharmaceuticals, cosmetics, and polymers. This white paper aims to provide an overview of aliphatic carboxylic acids, including their properties, applications, and production methods.
Properties:
Aliphatic carboxylic acids have several important properties, including their solubility, acidity, and boiling points. The solubility of aliphatic carboxylic acids in water depends on the length of their carbon chain and the presence of polar functional groups. Short-chain aliphatic carboxylic acids, such as formic acid and acetic acid, are highly soluble in water, while longer-chain acids, such as stearic acid, are less soluble.
Aliphatic carboxylic acids are also acidic due to the presence of the carboxyl group. They can donate a hydrogen ion (H+) to form a carboxylate ion (-COO-), which makes them weak acids. The acidity of aliphatic carboxylic acids increases with the length of the carbon chain.
The boiling points of aliphatic carboxylic acids also depend on the length of their carbon chain. Short-chain acids have lower boiling points than longer-chain acids due to the weaker intermolecular forces between molecules.
Applications:
Aliphatic carboxylic acids are used in a variety of applications in different industries. Some of the most common applications of aliphatic carboxylic acids are as follows:
- Food industry: Aliphatic carboxylic acids, such as acetic acid and citric acid, are widely used as food additives for their acidity and flavor-enhancing properties. They are commonly used in soft drinks, candies, and baked goods.
- Pharmaceutical industry: Aliphatic carboxylic acids, such as salicylic acid and acetylsalicylic acid (aspirin), are used in the production of pharmaceuticals due to their anti-inflammatory and analgesic properties.
- Cosmetics industry: Aliphatic carboxylic acids, such as lauric acid and stearic acid, are used in the production of soaps, lotions, and creams as emulsifiers and surfactants.
- Polymer industry: Aliphatic carboxylic acids, such as lactic acid, are used in the production of biodegradable polymers, such as poly(lactic acid) (PLA), which are eco-friendly alternatives to traditional plastics.
Production methods:
Aliphatic carboxylic acids can be produced through various methods, including oxidation of primary alcohols, hydrocarbons, and aldehydes, as well as through the carboxylation of organometallic compounds. The most common methods for the production of aliphatic carboxylic acids are:
- Oxidation of primary alcohols: Primary alcohols can be oxidized to form aldehydes, which can then be further oxidized to form carboxylic acids. The oxidation can be carried out using various oxidizing agents, such as potassium permanganate, chromic acid, and nitric acid.
- Carboxylation of organometallic compounds: Organometallic compounds, such as Grignard reagents, can be carboxylated to form carboxylic acids. The reaction involves the addition of carbon dioxide to the organometallic compound in the presence of a catalyst, such as copper or nickel.
Conclusion:
Aliphatic carboxylic acids are important organic compounds with a carboxyl group (-COOH) attached to an aliphatic carbon chain. They have several important properties, including their solubility, acidity, and boiling points, which make them useful in various industries, including food, pharmaceuticals, cosmetics, and polymers.
Aliphatic carboxylic acids are used as food additives, in the production of pharmaceuticals, soaps, lotions, and creams, and in the production of biodegradable polymers. They can be produced through various methods, including the oxidation of primary alcohols and the carboxylation of organometallic compounds.
Overall, aliphatic carboxylic acids play an important role in various industries and will continue to be a valuable organic compound for many applications in the future.
