Gene Therapy
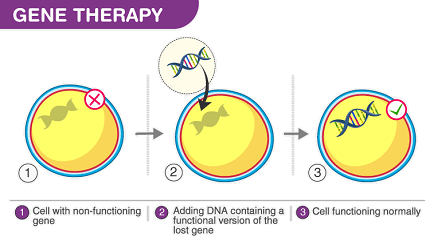
Gene therapy is a therapeutic approach that aims to treat or prevent diseases by modifying or manipulating the genetic material (DNA or RNA) of an individual. It involves introducing functional genes, modifying existing genes, or silencing abnormal genes to correct genetic defects or restore normal cellular functions.
The process of gene therapy typically involves the following steps:
- Identification of the target gene: The specific gene or genes responsible for a particular disease or condition are identified.
- Gene delivery: The therapeutic genes are delivered into the target cells using various delivery systems. Common methods include viral vectors (e.g., modified viruses) or non-viral vectors (e.g., liposomes or nanoparticles). These vectors act as vehicles to transport the therapeutic genes into the cells.
- Gene expression or editing: Once the therapeutic genes are delivered into the target cells, they can be designed to function in different ways:
- Gene addition: In this approach, the therapeutic gene is added to the target cells, supplementing the missing or defective gene. The introduced gene is then expressed, producing the functional protein.
- Gene editing: With the help of advanced gene editing techniques like CRISPR-Cas9, specific genes can be modified or edited to correct mutations or remove undesirable gene sequences.
- Gene silencing: In certain cases, the goal is to inhibit the expression of specific genes that contribute to disease. This can be achieved through techniques like RNA interference (RNAi), where small RNA molecules are used to silence the expression of specific genes.
- Monitoring and evaluation: After gene therapy, the treated cells or tissues are monitored to assess the effectiveness of the treatment and any potential side effects. Various molecular and cellular techniques are used to evaluate gene expression, protein production, and overall therapeutic outcomes.
Gene therapy has shown promise in treating a range of genetic disorders, including inherited diseases, immune disorders, and certain types of cancer. It is also being explored as a potential treatment for non-genetic diseases by targeting specific genes or pathways involved in disease progression.
However, gene therapy is still a developing field, and there are challenges and limitations that need to be addressed. These include ensuring the safety and long-term stability of gene expression, optimizing gene delivery methods, minimizing immune responses to viral vectors, and addressing ethical and regulatory considerations.
Ongoing research and advancements in gene therapy hold great potential for the development of innovative treatments and personalized medicine approaches to combat a wide range of diseases.
The syllabus for the Biology section of an advanced course at AIIMS (All India Institute of Medical Sciences) may cover various topics related to gene therapy. Gene therapy is a rapidly evolving field in medicine that focuses on the use of genes to treat or prevent diseases. Here is a general outline of the topics that may be included in the syllabus:
- Introduction to Gene Therapy:
- Definition, historical background, and scope of gene therapy.
- Overview of the basic principles and techniques used in gene therapy.
- Molecular Biology and Genetic Engineering:
- Fundamentals of DNA, RNA, and protein synthesis.
- Techniques for DNA manipulation, including recombinant DNA technology.
- Gene cloning and expression systems.
- Gene Delivery Systems:
- Different methods for gene delivery, such as viral vectors (retroviruses, adenoviruses, lentiviruses), non-viral vectors (plasmid DNA, nanoparticles), and others.
- Advantages and limitations of each delivery system.
- Gene Editing Techniques:
- Introduction to gene editing techniques, including CRISPR-Cas9 and other emerging technologies.
- Applications of gene editing in gene therapy.
- Types of Gene Therapy:
- Somatic gene therapy: Introduction, strategies, and applications.
- Germline gene therapy: Ethical considerations and controversies.
- Diseases and Applications:
- Gene therapy for monogenic disorders, such as cystic fibrosis, hemophilia, muscular dystrophy, and sickle cell anemia.
- Gene therapy for cancer: Tumor suppressor genes, oncolytic viruses, and immunotherapy approaches.
- Gene therapy for neurodegenerative disorders, cardiovascular diseases, and other conditions.
- Challenges and Future Directions:
- Regulatory and ethical considerations in gene therapy.
- Safety concerns and adverse effects.
- Emerging trends and future prospects in gene therapy research.
It’s important to note that the actual syllabus may vary depending on the specific curriculum of the course and any updates in the field of gene therapy. It’s always recommended to refer to the official course materials and consult with the instructors for the most accurate and up-to-date information.
What is Required Biology syllabus Gene Therapy
The specific topics covered in the biology syllabus for gene therapy may vary depending on the educational institution or course. However, here is a general outline of the key concepts and areas that are typically covered:
- Introduction to Gene Therapy:
- Definition, historical background, and basic principles of gene therapy.
- Importance and potential applications of gene therapy in medicine.
- Basics of Molecular Biology:
- Structure and function of DNA, RNA, and proteins.
- DNA replication, transcription, and translation.
- Gene expression and regulation.
- Genetic Disorders and Gene Therapy:
- Overview of different types of genetic disorders, including monogenic disorders and multifactorial disorders.
- Understanding the genetic basis of diseases and the role of specific genes.
- Importance of gene therapy in treating genetic disorders.
- Gene Delivery Systems:
- Different methods and vectors used for gene delivery in gene therapy, such as viral vectors (retroviruses, adenoviruses, lentiviruses), non-viral vectors (liposomes, nanoparticles), and physical methods (electroporation, gene gun).
- Advantages, limitations, and safety considerations of each gene delivery system.
- Gene Editing Techniques:
- Introduction to gene editing technologies, including CRISPR-Cas9 and other targeted genome editing tools.
- Understanding the mechanisms and applications of gene editing in gene therapy.
- Strategies and Approaches in Gene Therapy:
- Somatic gene therapy: Principles, strategies, and applications.
- Germline gene therapy: Ethical considerations and current debates.
- Ex vivo and in vivo gene therapy approaches.
- Clinical Applications of Gene Therapy:
- Gene therapy for specific genetic disorders, such as cystic fibrosis, hemophilia, muscular dystrophy, and sickle cell anemia.
- Gene therapy for cancer and other acquired diseases.
- Challenges and ongoing research in developing effective gene therapy treatments.
- Safety, Ethics, and Regulatory Considerations:
- Safety concerns associated with gene therapy, including immune responses and off-target effects.
- Ethical considerations, including informed consent, privacy, and equitable access to gene therapies.
- Regulatory frameworks and guidelines for gene therapy research and clinical trials.
It’s important to note that the actual syllabus may vary depending on the institution, course level, and specific requirements. It’s recommended to consult the official syllabus or course materials provided by the educational institution to obtain the most accurate and up-to-date information.
When is Required Biology syllabus Gene Therapy
The inclusion of gene therapy in the biology syllabus can vary depending on the educational institution, curriculum, and level of study. Gene therapy is a specialized topic within the field of biology, and it may be covered in various contexts or courses such as molecular biology, genetics, biotechnology, or advanced medical sciences. The specific timing of when gene therapy is taught can vary.
In general, gene therapy is often covered at the undergraduate or postgraduate level in biology or related disciplines. It may be included in advanced courses that focus on molecular biology, genetic engineering, or biomedical sciences. The timing of when gene therapy is taught can differ between institutions, but it is commonly covered after students have gained a foundational understanding of genetics, molecular biology, and cellular processes.
It’s recommended to check the course curriculum or syllabus provided by your educational institution to determine when gene therapy is specifically included in the biology syllabus. This will help you understand when you can expect to study and learn about gene therapy in your academic program.
Where is Required Biology syllabus Gene Therapy
The inclusion of gene therapy in the biology syllabus can vary depending on the educational institution and the specific curriculum being followed. In most cases, gene therapy is covered as part of specialized courses or modules that focus on molecular biology, genetics, or biotechnology. These courses are typically offered at the undergraduate or postgraduate level in biology or related fields.
Gene therapy may be included in the syllabus of the following types of courses:
- Molecular Biology: Gene therapy concepts and techniques are often covered as part of molecular biology courses. These courses delve into the structure and function of genes, gene expression, DNA replication, and genetic engineering techniques.
- Genetics: Gene therapy is directly related to the field of genetics. Genetics courses cover the principles of inheritance, gene regulation, and genetic disorders. Gene therapy is often discussed in the context of genetic disorders and the potential for using genetic interventions to treat them.
- Biotechnology/Bioengineering: Gene therapy is a prominent application of biotechnology and bioengineering. Courses in these fields may cover gene therapy as a specialized topic, focusing on gene delivery systems, gene editing techniques, and the ethical and regulatory aspects of gene therapy.
- Advanced Medical Sciences: In advanced medical sciences programs, gene therapy may be included as part of specialized courses related to molecular medicine, regenerative medicine, or personalized medicine. These courses explore the cutting-edge research and clinical applications of gene therapy in treating various diseases.
It’s important to note that the specific placement of gene therapy within the biology syllabus can vary between institutions. To know the exact location of gene therapy in your specific biology syllabus, it is recommended to refer to the curriculum or syllabus provided by your educational institution or consult with your instructors or academic advisors. They will be able to provide you with the most accurate information regarding the inclusion and placement of gene therapy in your biology program.
How is Required Biology syllabus Gene Therapy
The way gene therapy is included in the biology syllabus can vary depending on the educational institution, curriculum, and level of study. However, here is a general overview of how gene therapy may be taught in the biology syllabus:
- Introduction to Gene Therapy:
- Overview of gene therapy as a therapeutic approach.
- Historical background and development of gene therapy.
- Basic principles and concepts underlying gene therapy.
- Genetic Basis of Diseases:
- Understanding the genetic basis of diseases and the role of specific genes in disease development.
- Different types of genetic disorders and their impact on health.
- Identification and characterization of disease-causing genes.
- Molecular Biology Techniques:
- Key molecular biology techniques and methodologies used in gene therapy research.
- DNA cloning, recombinant DNA technology, and gene expression analysis.
- Basic principles of gene editing techniques, such as CRISPR-Cas9.
- Gene Delivery Systems:
- Different methods and vectors used for gene delivery in gene therapy.
- Viral vectors (retroviruses, adenoviruses, lentiviruses) and non-viral vectors (liposomes, nanoparticles).
- Advantages, limitations, and safety considerations of gene delivery systems.
- Gene Editing Techniques and Applications:
- In-depth understanding of gene editing techniques, such as CRISPR-Cas9, TALENs, or zinc finger nucleases.
- Applications of gene editing in gene therapy for correcting genetic mutations or modifying gene expression.
- Ethical and regulatory considerations of gene editing technologies.
- Clinical Applications of Gene Therapy:
- Case studies and examples of gene therapy used for treating specific genetic disorders or diseases.
- Gene therapy approaches for monogenic disorders, cancer, neurodegenerative diseases, and other conditions.
- Successes, challenges, and future prospects of gene therapy in the clinical setting.
- Safety and Ethical Considerations:
- Safety concerns associated with gene therapy, including immune responses, off-target effects, and long-term consequences.
- Ethical considerations in gene therapy research and clinical applications.
- Regulatory frameworks and guidelines governing gene therapy research and clinical trials.
- Current Research and Future Directions:
- Recent advances and ongoing research in gene therapy.
- Emerging trends and technologies in the field of gene therapy.
- Potential future applications and challenges in implementing gene therapy in clinical practice.
It’s important to note that the specific content and depth of gene therapy in the biology syllabus can vary between educational institutions and courses. The above outline provides a general framework of the topics and concepts typically covered in the study of gene therapy within a biology curriculum. For detailed and accurate information about the biology syllabus for gene therapy, it’s recommended to refer to the curriculum or syllabus provided by your educational institution.
Nomenclature of Biology syllabus Gene Therapy
The nomenclature or naming of the biology syllabus for gene therapy can vary depending on the educational institution and the specific course or program. However, here are some possible nomenclatures that might be used to represent the biology syllabus for gene therapy:
- “Gene Therapy” or “Gene Therapy: Principles and Applications”
- “Molecular Biology and Gene Therapy”
- “Advanced Topics in Genetics: Gene Therapy”
- “Biotechnology and Gene Therapy”
- “Genetic Engineering and Gene Therapy”
- “Gene Therapy in Medicine”
- “Applications of Gene Therapy in Biology”
- “Genomics and Gene Therapy”
These are just examples, and the actual nomenclature used by educational institutions may differ. It’s important to refer to the official course catalog, curriculum, or syllabus provided by your specific educational institution to obtain the accurate and official nomenclature for the biology syllabus on gene therapy.
Case Study on Biology syllabus Gene Therapy
Case Study: Gene Therapy for Severe Combined Immunodeficiency (SCID)
Introduction: Severe Combined Immunodeficiency (SCID) is a rare genetic disorder characterized by a severe impairment of the immune system, leaving affected individuals highly susceptible to life-threatening infections. One of the most well-known cases of successful gene therapy involves the treatment of SCID caused by a deficiency of the enzyme adenosine deaminase (ADA). This case study focuses on the pioneering use of gene therapy to treat SCID-ADA.
Background: ADA-deficient SCID is caused by mutations in the ADA gene, which leads to a lack of functional adenosine deaminase enzyme. Without this enzyme, toxic metabolites accumulate in the body, primarily affecting the development and function of immune cells. Traditional treatments for SCID-ADA included enzyme replacement therapy and bone marrow transplantation. However, these approaches had limitations and were not always successful in providing a long-term cure.
Case Study Details: In the early 2000s, a groundbreaking gene therapy trial was conducted to treat SCID-ADA. The study involved a small group of children with ADA-deficient SCID who had limited or no treatment options. The gene therapy approach aimed to restore the function of the ADA gene in the patients’ cells, particularly in hematopoietic stem cells (HSCs) that give rise to immune cells.
- Collection of Patient’s HSCs: HSCs were obtained from the patients’ bone marrow or peripheral blood.
- Ex Vivo Gene Transfer: The patients’ HSCs were cultured and then genetically modified outside the body using a retroviral vector carrying the functional ADA gene. The retroviral vector integrated the ADA gene into the genome of the patients’ HSCs.
- Conditioning Regimen: Prior to the infusion of genetically modified HSCs, the patients received a conditioning regimen involving low-dose chemotherapy to partially deplete their existing bone marrow cells. This created space for the newly modified HSCs to engraft and regenerate the immune system.
- Infusion of Gene-Modified HSCs: The patients received intravenous infusion of their own gene-modified HSCs, allowing the cells to travel to the bone marrow and establish themselves.
- Immune System Reconstitution: Over time, the gene-modified HSCs differentiated and produced functional immune cells, including T cells, B cells, and natural killer (NK) cells, with restored ADA enzyme activity.
Results: The initial results of the gene therapy trial were encouraging. The infused gene-modified HSCs successfully engrafted and restored immune cell function in the treated patients. Long-term follow-up studies showed sustained immune reconstitution and improved clinical outcomes. The patients demonstrated reduced susceptibility to infections, improved growth, and enhanced quality of life.
Lessons Learned and Future Perspectives: The successful application of gene therapy for SCID-ADA marked a significant milestone in the field. However, this case study also highlighted important considerations and challenges for gene therapy:
- Safety Concerns: The initial success was marred by the occurrence of leukemia in a few patients due to insertional mutagenesis, where the retroviral vector disrupted a gene associated with cancer development. This emphasized the need for careful monitoring and refinement of gene delivery systems to minimize potential adverse effects.
- Advances in Vector Systems: Subsequent research has focused on the development of safer and more efficient vector systems, such as lentiviral vectors and gene editing technologies like CRISPR-Cas9, to overcome the limitations associated with retroviral vectors.
- Broadening Applications: Gene therapy has expanded beyond SCID-ADA to target other forms of SCID and genetic disorders. It has shown promise in treating a range of diseases, including inherited disorders, cancer, and certain acquired conditions.
- Regulatory Approval and Accessibility: Gene therapy has gained regulatory approval for specific indications, and efforts are being made to improve its accessibility, cost-effectiveness, and availability to patients worldwide.
Conclusion: The case study of gene therapy for SCID-ADA illustrates the potential of gene therapy as a transformative approach for treating genetic disorders. While challenges remain, ongoing research and technological advancements continue to enhance the safety and efficacy of gene therapy, paving the way for its broader application in the future.
White paper on Biology syllabus Gene Therapy
Title: Advancements in Gene Therapy: Transforming Medicine for Genetic Disorders
Abstract: Gene therapy, a groundbreaking approach in the field of medicine, holds immense promise for the treatment of genetic disorders. This white paper provides an overview of gene therapy, discussing its principles, recent advancements, challenges, and future prospects. It highlights the transformative impact of gene therapy on the management and potential cure of genetic disorders, ultimately improving patients’ quality of life. The paper also explores the ethical, regulatory, and commercial considerations surrounding gene therapy, emphasizing the need for continued research and collaboration to unlock its full potential.
- Introduction
- Definition and principles of gene therapy.
- Historical background and milestones in the development of gene therapy.
- Importance of gene therapy in addressing the unmet medical needs of individuals with genetic disorders.
- Gene Therapy Techniques
- Overview of different gene therapy approaches, including gene addition, gene editing, and gene silencing.
- Key techniques and tools utilized in gene therapy, such as viral vectors, non-viral vectors, and gene editing technologies.
- Advantages and limitations of various gene therapy techniques.
- Applications of Gene Therapy
- Treatment of monogenic disorders, including examples such as cystic fibrosis, hemophilia, muscular dystrophy, and inherited retinal diseases.
- Gene therapy for acquired diseases, such as cancer, cardiovascular diseases, and neurodegenerative disorders.
- Potential applications in regenerative medicine and personalized medicine.
- Recent Advancements in Gene Therapy
- Clinical successes and breakthroughs in gene therapy trials.
- Improved vector systems, including adeno-associated viruses (AAVs) and lentiviral vectors.
- Advancements in gene editing technologies, such as CRISPR-Cas9 and base editing.
- Targeted gene therapies and tissue-specific delivery approaches.
- Challenges and Considerations
- Safety concerns, including immune responses and off-target effects.
- Ethical considerations related to germline gene therapy and genetic enhancement.
- Regulatory frameworks and guidelines governing gene therapy research and clinical trials.
- Commercialization and market access challenges for gene therapy products.
- Future Perspectives
- Emerging trends and technologies in gene therapy research.
- Potential expansion of gene therapy to more genetic disorders and broader patient populations.
- Integration of gene therapy with other treatment modalities, such as immunotherapy and precision medicine.
- Advancements in manufacturing processes and scalability of gene therapy products.
- Conclusion
- Summary of the transformative potential of gene therapy in the treatment of genetic disorders.
- Call for continued research, collaboration, and investment to address challenges and unlock the full potential of gene therapy.
- The importance of equitable access to gene therapy for patients worldwide.
This white paper aims to provide a comprehensive overview of gene therapy, showcasing its significant contributions to the field of medicine. It serves as a valuable resource for healthcare professionals, researchers, policymakers, and stakeholders interested in understanding the current state and future prospects of gene therapy in transforming the landscape of genetic disorder treatment.