Bubbles: Bubbles are pockets of gas enclosed by a thin layer of liquid. They can form in a liquid due to a variety of reasons, such as agitation, heating, or the release of dissolved gases. Bubbles are important in many industrial processes and are also a fascinating subject of study in fields such as chemistry, physics, and engineering.
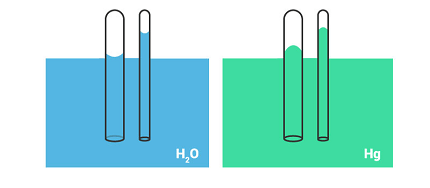
Capillary Rise: Capillary rise is the phenomenon of a liquid rising up a narrow tube or capillary due to surface tension and adhesion between the liquid and the tube. This effect can be observed in many everyday situations, such as when a paper towel soaks up a spill or when water is drawn up through the roots of a plant.
The height to which the liquid rises in the capillary is determined by the balance between the upward capillary force and the downward gravitational force. The capillary force depends on the surface tension of the liquid, the diameter of the capillary, and the angle of contact between the liquid and the capillary wall.
Both bubbles and capillary rise are examples of surface tension phenomena and can be explained by the same principles of intermolecular forces and fluid dynamics.
What is Required Bubbles and Capillary rise
The requirements for bubbles and capillary rise are different:
For bubbles, you typically need a liquid that can dissolve gas or has gas present in it. The gas can be released due to agitation, temperature changes, or pressure changes, resulting in the formation of bubbles. The size and stability of bubbles depend on factors such as the type of gas, the concentration of gas in the liquid, and the surface tension of the liquid.
For capillary rise, you need a narrow tube or capillary that is immersed in a liquid. The liquid should have a higher surface tension and adhesion to the tube than cohesion within the liquid itself. This allows the liquid to rise up the capillary due to the cohesive forces between the liquid molecules and the adhesive forces between the liquid and the capillary wall. The height of the capillary rise depends on the properties of the liquid, the diameter of the capillary, and the angle of contact between the liquid and the capillary wall.
In summary, the formation of bubbles requires gas present in a liquid and factors that affect their stability, while capillary rise requires a narrow tube immersed in a liquid with specific surface tension and adhesion properties.
When is Required Bubbles and Capillary rise
Bubbles and capillary rise can be observed in various situations and have different applications. Here are a few examples:
Bubbles:
- In carbonated drinks, bubbles of carbon dioxide gas are formed due to the high pressure in the bottle or can.
- In boiling water, bubbles of water vapor form at the bottom of the pot and rise to the surface, carrying heat away from the liquid.
- In chemical reactions, bubbles may be formed as a byproduct of the reaction, which can have implications for the reaction efficiency or safety.
Capillary rise:
- In plants, capillary action helps to transport water and nutrients from the roots to the leaves.
- In paper towels or sponges, capillary rise allows for the absorption and retention of liquids.
- In medical devices, such as microfluidic systems, capillary forces can be used to control the flow and manipulation of small volumes of liquids.
In general, bubbles and capillary rise are important phenomena in fields such as physics, chemistry, biology, and engineering. Understanding the underlying principles and factors that affect these phenomena can have practical applications in a wide range of industries and technologies.
Where is Required Bubbles and Capillary rise
Bubbles and capillary rise can occur in a variety of settings and environments, including:
Bubbles:
- In oceans, lakes, and rivers, bubbles may form due to the release of gases from decaying matter or volcanic activity.
- In chemical manufacturing processes, bubbles may be used to mix and react different chemicals.
- In biomedical research, bubbles can be used in ultrasound imaging or to deliver drugs to specific tissues or organs.
Capillary rise:
- In plants, capillary action is essential for the uptake of water and nutrients from the soil.
- In porous materials, such as soils, rocks, and concrete, capillary rise can influence the transport of water and other fluids.
- In microfluidic devices, capillary forces can be used to move and manipulate small volumes of liquids in a controlled manner.
In general, bubbles and capillary rise are ubiquitous phenomena that can be found in a wide range of natural and artificial systems. Understanding how these phenomena operate in different contexts can provide valuable insights into the behavior of fluids and the dynamics of intermolecular forces.
How is Required Bubbles and Capillary rise
Bubbles and capillary rise can be explained by the principles of surface tension, adhesion, and cohesion.
For bubbles, the formation and stability of bubbles depend on the properties of the liquid and gas involved. When a gas is introduced into a liquid, the gas molecules dissolve and become evenly distributed throughout the liquid. If the concentration of gas in the liquid exceeds the equilibrium solubility, the excess gas molecules will begin to form bubbles. The surface tension of the liquid causes the bubble to form a spherical shape, while the gas inside the bubble exerts pressure on the liquid surface to maintain its shape. The stability of the bubble depends on factors such as the gas concentration, the size of the bubble, and the surface tension of the liquid.
For capillary rise, the upward movement of the liquid in a narrow tube or capillary is due to the cohesive forces between the liquid molecules and the adhesive forces between the liquid and the capillary wall. The surface tension of the liquid causes the liquid to form a meniscus at the surface of the tube, which creates a concave or convex shape depending on the relative magnitudes of the cohesive and adhesive forces. The height of the capillary rise is determined by the balance between the upward capillary force and the downward gravitational force, which depends on the properties of the liquid, the diameter of the capillary, and the angle of contact between the liquid and the capillary wall.
In summary, the formation of bubbles and capillary rise can be explained by the interplay of surface tension, adhesion, and cohesion forces, which operate at the interface between the liquid and the surrounding medium. Understanding these principles is essential for predicting and controlling the behavior of fluids in a variety of natural and artificial systems.
Structures of Bubbles and Capillary rise
The structures of bubbles and capillary rise are different due to the underlying principles that govern their formation.
Bubbles: Bubbles are formed when gas is introduced into a liquid, and the gas molecules become trapped by the surface tension of the liquid. The resulting structure is a spherical shape with a thin film of liquid surrounding the gas inside the bubble. The film thickness varies depending on factors such as the surface tension of the liquid and the size of the bubble. As the gas inside the bubble expands or contracts, the film thickness changes, which can affect the stability and lifetime of the bubble.
Capillary rise: Capillary rise occurs when a liquid is placed in a narrow tube or capillary, and the cohesive forces between the liquid molecules and the adhesive forces between the liquid and the capillary wall cause the liquid to rise up the tube. The resulting structure is a meniscus, which is the curved surface of the liquid at the interface with the air. The shape of the meniscus depends on the balance between the cohesive and adhesive forces, which can result in a concave or convex shape. The height of the capillary rise is determined by the diameter of the capillary and the properties of the liquid.
In summary, the structures of bubbles and capillary rise are characterized by different shapes and surface structures. Bubbles are spherical structures with a thin film of liquid surrounding the gas inside, while capillary rise produces a meniscus shape due to the cohesive and adhesive forces between the liquid and the capillary wall.
Case Study on Bubbles and Capillary rise
A case study on the use of bubbles and capillary rise can be found in the field of microfluidics. Microfluidics is the study and manipulation of fluids in small volumes, typically on the scale of microliters or nanoliters. In microfluidic devices, capillary rise and bubbles can be used for a variety of applications, such as pumping, mixing, and sample handling.
One example of the use of capillary rise in microfluidics is in paper-based analytical devices (PADs). PADs are low-cost, portable, and easy-to-use diagnostic tools that use capillary forces to drive fluids through paper channels. The channels are typically hydrophilic, meaning they attract water, while the surrounding paper is hydrophobic, meaning it repels water. When a liquid sample is placed on the channel, it is drawn through the channel by capillary rise, and the resulting pattern can be used to detect the presence or absence of specific analytes.
Another example of the use of bubbles in microfluidics is in microfluidic droplet generation. Droplet generation is the process of creating small, uniform droplets of a liquid, typically in the range of microliters or nanoliters. In microfluidics, droplet generation is often achieved by using gas bubbles to break up a continuous stream of liquid into discrete droplets. The bubbles act as templates for the droplets, providing a well-defined size and shape. The resulting droplets can be used for a variety of applications, such as high-throughput screening, drug delivery, and bioreactors.
In conclusion, the use of bubbles and capillary rise in microfluidics is a growing area of research and development, with a wide range of applications in fields such as diagnostics, drug delivery, and bioreactors. By understanding the principles of surface tension, adhesion, and cohesion, researchers and engineers can design and optimize microfluidic devices to achieve specific fluidic behaviors and functionalities.
White paper on Bubbles and Capillary rise
Here is a white paper on Bubbles and Capillary rise:
Introduction
Bubbles and capillary rise are two phenomena that occur at the interface between a liquid and a gas or solid. These phenomena are governed by the principles of surface tension, adhesion, and cohesion, and have important applications in a variety of fields, including chemistry, physics, biology, and engineering. In this white paper, we will discuss the underlying principles of bubbles and capillary rise, as well as their applications in various fields.
Bubbles
Bubbles are spherical pockets of gas that are trapped within a liquid. The formation and stability of bubbles depend on the properties of the liquid and the gas involved. When a gas is introduced into a liquid, the gas molecules dissolve and become evenly distributed throughout the liquid. If the concentration of gas in the liquid exceeds the equilibrium solubility, the excess gas molecules will begin to form bubbles. The surface tension of the liquid causes the bubble to form a spherical shape, while the gas inside the bubble exerts pressure on the liquid surface to maintain its shape. The stability of the bubble depends on factors such as the gas concentration, the size of the bubble, and the surface tension of the liquid.
Bubbles have a wide range of applications in various fields. For example, in the food and beverage industry, bubbles are used to create foam and carbonation. In the medical field, bubbles are used for diagnostic and therapeutic purposes, such as contrast agents for ultrasound imaging and drug delivery vehicles. In the chemical and petroleum industries, bubbles are used for gas-liquid reactions and separations.
Capillary rise
Capillary rise is the upward movement of a liquid in a narrow tube or capillary due to the cohesive forces between the liquid molecules and the adhesive forces between the liquid and the capillary wall. The surface tension of the liquid causes the liquid to form a meniscus at the surface of the tube, which creates a concave or convex shape depending on the relative magnitudes of the cohesive and adhesive forces. The height of the capillary rise is determined by the balance between the upward capillary force and the downward gravitational force, which depends on the properties of the liquid, the diameter of the capillary, and the angle of contact between the liquid and the capillary wall.
Capillary rise has a wide range of applications in various fields. For example, in the field of microfluidics, capillary rise is used in paper-based analytical devices for diagnostic testing. In the field of materials science, capillary rise is used to study the wetting behavior of liquids on surfaces. In the field of soil science, capillary rise is used to understand the movement of water in soil.
Conclusion
Bubbles and capillary rise are two phenomena that occur at the interface between a liquid and a gas or solid. The principles of surface tension, adhesion, and cohesion govern these phenomena, and they have important applications in various fields, including chemistry, physics, biology, and engineering. By understanding the underlying principles of bubbles and capillary rise, researchers and engineers can design and optimize materials, devices, and processes for specific applications.
