The Carbylamine reaction, also known as the isocyanide test or Hofmann’s isocyanide test, is a chemical test used to detect the presence of primary amines in a given compound. The test was discovered by August Wilhelm von Hofmann in 1861 and is based on the reaction of primary amines with chloroform and a strong base, usually sodium hydroxide (NaOH) or potassium hydroxide (KOH), to produce foul-smelling isocyanides, also known as carbylamines.
The reaction proceeds as follows:
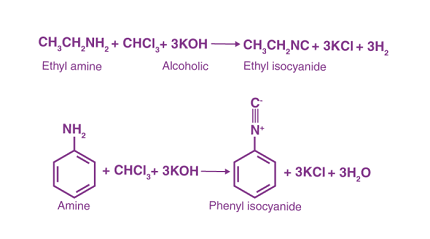
RNH2 + CHCl3 + 3KOH → RNC + 3KCl + 3H2O
The carbylamine produced in the reaction has a strong, disagreeable odor similar to that of mustard gas. The presence of this odor confirms the presence of a primary amine in the original compound.
The Carbylamine reaction is commonly used in organic chemistry for the identification and characterization of primary amines. However, it should be noted that the test is not specific for primary amines and can also detect other compounds, such as ureas and thioureas, that contain the isocyanide functional group.
What is Required Amines Carbylamine reaction
The Carbylamine reaction, also known as the isocyanide test or Hofmann’s isocyanide test, is a chemical test used to detect the presence of primary amines in a given compound. In this reaction, primary amines react with chloroform and a strong base, usually sodium hydroxide (NaOH) or potassium hydroxide (KOH), to produce foul-smelling isocyanides, also known as carbylamines.
Therefore, primary amines are required for the Carbylamine reaction to occur. Secondary and tertiary amines do not undergo this reaction under normal conditions. However, some substituted primary amines may not give the Carbylamine reaction due to steric hindrance or electronic factors.
When is Required Amines Carbylamine reaction
The Carbylamine reaction is used to detect the presence of primary amines in a given compound. It is commonly used in organic chemistry for the identification and characterization of primary amines. The test is usually performed by mixing the unknown compound with chloroform and a strong base, such as sodium hydroxide or potassium hydroxide, and heating the mixture. If a primary amine is present, a foul-smelling isocyanide, or carbylamine, will be produced.
The Carbylamine reaction is a useful tool in organic chemistry because primary amines are important functional groups that are found in many natural and synthetic compounds. The test can be used to identify primary amines in a wide range of compounds, including amino acids, peptides, proteins, and alkaloids. The reaction is also used in the production of isocyanides, which are important building blocks for the synthesis of a variety of organic compounds.
It should be noted that while the Carbylamine reaction is a useful test for primary amines, it is not specific for primary amines and can also detect other compounds, such as ureas and thioureas, that contain the isocyanide functional group. Additionally, some substituted primary amines may not give the Carbylamine reaction due to steric hindrance or electronic factors.
Where is Required Amines Carbylamine reaction
The Carbylamine reaction is a chemical test used to detect the presence of primary amines in a given compound. It is a commonly used test in organic chemistry, and it can be performed in any laboratory equipped with the necessary chemicals and equipment.
The reaction can be carried out in a small test tube or flask using readily available reagents, such as chloroform and a strong base, such as sodium hydroxide or potassium hydroxide. The test is usually performed by heating the mixture of the unknown compound, chloroform, and the strong base to produce a foul-smelling isocyanide, or carbylamine, if a primary amine is present.
The Carbylamine reaction is a simple and straightforward test that can be performed in any laboratory setting, making it a valuable tool for the identification and characterization of primary amines in a wide range of natural and synthetic compounds.
How is Required Amines Carbylamine reaction
The Carbylamine reaction, also known as the Hofmann’s isocyanide test, is a simple and straightforward chemical test used to detect the presence of primary amines in a given compound. The reaction is carried out by mixing the unknown compound with chloroform and a strong base, such as sodium hydroxide or potassium hydroxide, and heating the mixture.
The reaction proceeds as follows:
RNH2 + CHCl3 + 3KOH → RNC + 3KCl + 3H2O
Where RNH2 represents the primary amine.
If a primary amine is present, the reaction produces a foul-smelling isocyanide, or carbylamine, that can be detected by its characteristic odor. The carbylamine is formed due to the dehydration of the primary amine and chloroform, which produces an isocyanide intermediate that reacts with the base to form the final product.
The Carbylamine reaction is a useful test for primary amines because it is simple, fast, and sensitive. However, it is important to note that the test is not specific for primary amines and can also detect other compounds, such as ureas and thioureas, that contain the isocyanide functional group. Additionally, some substituted primary amines may not give the Carbylamine reaction due to steric hindrance or electronic factors.
Structures of Amines Carbylamine reaction
In the Carbylamine reaction, primary amines react with chloroform and a strong base, such as sodium hydroxide or potassium hydroxide, to produce foul-smelling isocyanides, also known as carbylamines. The reaction proceeds as follows:
RNH2 + CHCl3 + 3KOH → RNC + 3KCl + 3H2O
Where RNH2 represents the primary amine and RNC represents the isocyanide.
The structure of a primary amine is RNH2, where R represents an organic group. The structure of chloroform is CHCl3, and the strong base used in the reaction is typically sodium hydroxide (NaOH) or potassium hydroxide (KOH). The structure of the isocyanide produced in the reaction is RNC, where R represents the same organic group as in the primary amine.
The isocyanides produced in the Carbylamine reaction are typically foul-smelling liquids that have a strong, pungent odor. The odor is often described as resembling that of mustard gas or rotten fish. The reaction is a useful tool in organic chemistry for the identification and characterization of primary amines, and the foul-smelling isocyanides produced can be easily detected by their characteristic odor.
Case Study on Amines Carbylamine reaction
Here is an example case study that demonstrates the application of the Carbylamine reaction in organic chemistry:
Case Study:
A researcher is studying the chemical composition of a natural product extracted from a rare plant species. The researcher suspects that the natural product contains primary amines, but they need to confirm this using a chemical test. They decide to use the Carbylamine reaction to detect the presence of primary amines in the natural product.
The researcher begins by preparing a small sample of the natural product and mixing it with chloroform and potassium hydroxide. The mixture is heated, and a foul-smelling gas is produced, indicating that primary amines are present in the natural product. The researcher confirms the presence of primary amines by performing a control test using a known primary amine compound, which also produces the characteristic foul-smelling gas when subjected to the Carbylamine reaction.
The researcher then proceeds to identify and isolate the primary amine compounds in the natural product using a series of chemical separation and purification techniques. They analyze the purified compounds using spectroscopic methods such as mass spectrometry and nuclear magnetic resonance (NMR) to confirm their chemical structures and elucidate their functional groups.
The Carbylamine reaction was a valuable tool in this study because it provided a simple and reliable method for detecting the presence of primary amines in the natural product. The foul-smelling isocyanides produced in the reaction were easily detected by their characteristic odor, allowing the researcher to quickly and efficiently identify the primary amine compounds in the sample. The Carbylamine reaction was also useful because it is a relatively simple and inexpensive test that can be performed in any laboratory setting.
White paper on Amines Carbylamine reaction
Introduction:
The Carbylamine reaction, also known as the Hofmann’s isocyanide test, is a simple and reliable chemical test used to detect the presence of primary amines in a given compound. The reaction involves mixing the unknown compound with chloroform and a strong base, such as sodium hydroxide or potassium hydroxide, and heating the mixture. If a primary amine is present, the reaction produces a foul-smelling isocyanide, or carbylamine, that can be detected by its characteristic odor. In this white paper, we will explore the history, mechanism, applications, and limitations of the Carbylamine reaction.
History:
The Carbylamine reaction was first discovered by August Wilhelm von Hofmann, a German chemist, in 1858. Hofmann was studying the properties of primary amines when he noticed that some of the compounds produced a foul-smelling gas when heated with chloroform and a strong base. Hofmann hypothesized that the gas was an isocyanide, and he confirmed this by performing additional chemical tests. Hofmann’s discovery was a significant breakthrough in the field of organic chemistry, and the Carbylamine reaction became a widely used method for detecting primary amines.
Mechanism:
The Carbylamine reaction proceeds through a series of steps that involve the conversion of a primary amine to an isocyanide. The reaction mechanism is as follows:
RNH2 + CHCl3 + 3KOH → RNC + 3KCl + 3H2O
Where RNH2 represents the primary amine and RNC represents the isocyanide.
The reaction begins with the deprotonation of the primary amine by the strong base, producing an amide intermediate. The amide then reacts with chloroform to form an isocyanide intermediate. The isocyanide intermediate is highly reactive and reacts with the base to produce the final product, the foul-smelling isocyanide.
Applications:
The Carbylamine reaction has many applications in organic chemistry, including:
- Detection of primary amines: The Carbylamine reaction is a reliable method for detecting the presence of primary amines in a given compound. The foul-smelling isocyanides produced in the reaction are easily detected by their characteristic odor, making the Carbylamine reaction a valuable tool for identifying and characterizing primary amines.
- Identification of ureas and thioureas: The Carbylamine reaction can also detect the presence of compounds that contain the isocyanide functional group, such as ureas and thioureas.
- Synthesis of isocyanides: The Carbylamine reaction can be used to synthesize isocyanides from primary amines. This can be useful in organic synthesis for the preparation of isocyanide-containing compounds.
Limitations:
While the Carbylamine reaction is a useful method for detecting primary amines, it does have some limitations. These include:
- Lack of specificity: The Carbylamine reaction is not specific for primary amines and can also detect other compounds that contain the isocyanide functional group. This can lead to false positives in some cases.
- Substituent effects: The reaction may not work for some substituted primary amines due to steric hindrance or electronic factors.
- Toxicity: Chloroform is a toxic substance and should be used with caution in the Carbylamine reaction.
Conclusion:
The Carbylamine reaction is a valuable tool in organic chemistry for the detection and identification of primary amines. The reaction is simple, reliable, and relatively inexpensive, making it a useful technique in many applications. However, it has limitations, such as lack of specificity and toxicity, which must be taken into account when using the reaction. Despite these limitations, the Carbylamine reaction remains a valuable method for identifying and characterizing primary amines in both research and industrial settings.
