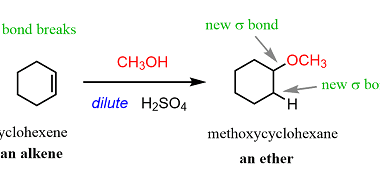
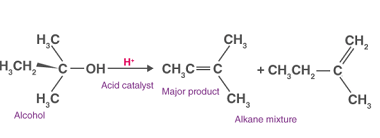
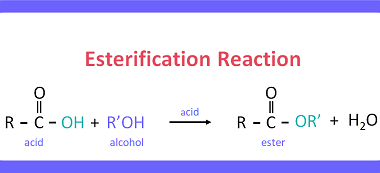
Carboxylic Acids
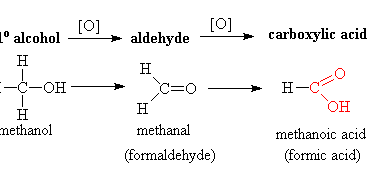
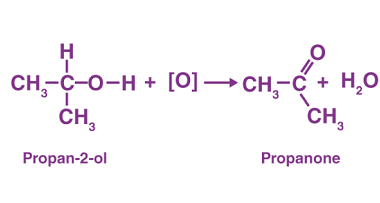
Carboxylic acids are organic compounds that contain a carboxyl functional group (-COOH). This functional group consists of a carbonyl group (C=O) and a hydroxyl group (-OH) bonded to the same carbon atom. The general formula for carboxylic acids is R-COOH, where R represents a hydrocarbon chain or a hydrogen atom. Carboxylic acids can be classified…