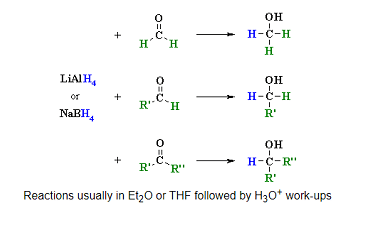
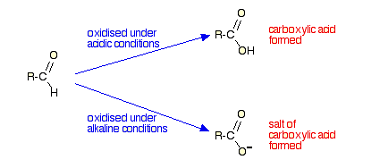
Reduction
Reduction of aldehydes and ketones is a common reaction in organic chemistry. The most commonly used reducing agents for this purpose are sodium borohydride (NaBH4) and lithium aluminum hydride (LiAlH4). Reduction of aldehydes: Aldehydes can be reduced to primary alcohols using either NaBH4 or LiAlH4 in the presence of an acid catalyst such as HCl.…