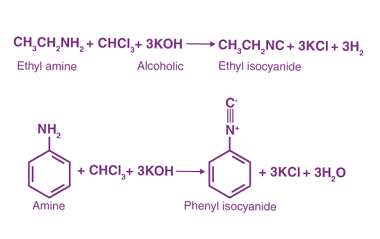
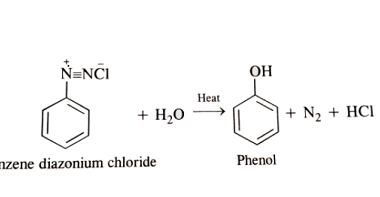
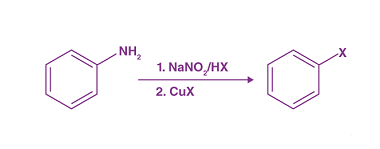
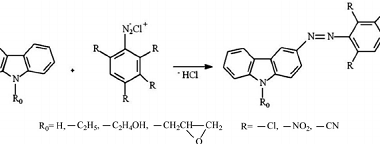
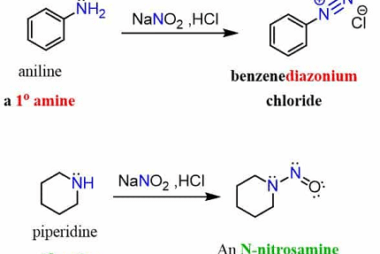
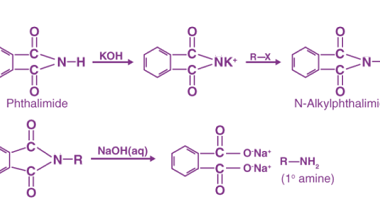
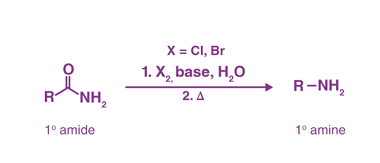Acylation reactions
Acylation reactions are chemical reactions in which an acyl group (-COCH3) is added to a molecule. The acyl group can be derived from an acid chloride (RCOCl), an anhydride (RCOOR), or a carboxylic acid (RCOOH) with an activating agent such as DCC (dicyclohexylcarbodiimide) or SOCl2 (thionyl chloride). One common type of acylation reaction is the…