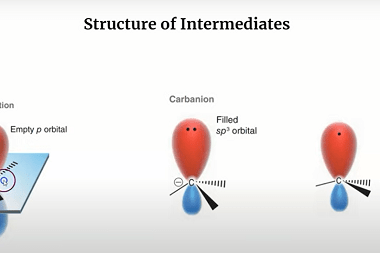
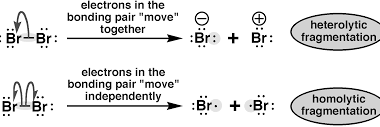
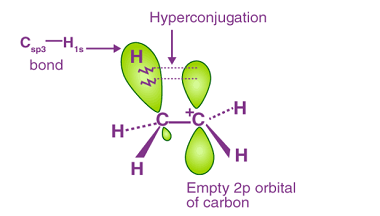
Carbanions and Free radicals
Carbanions and free radicals are two types of reactive intermediates that are commonly encountered in organic chemistry. A carbanion is an anionic species with a negatively charged carbon atom. It is formed when a carbon atom gains an electron pair and becomes negatively charged. Carbanions are typically very reactive and are often involved in nucleophilic…